✔ 100% Authentic Product
👁️ Currently Viewing 4101
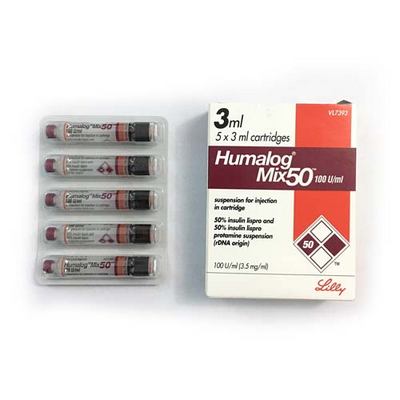
Generic Name: Insulin Lispro (25%/50%) + Insulin Lispro Protamine (75%/50%) mix
Company Name: Eli Lilly and Company
🚚 তাপমাত্রা নিয়ন্ত্রিত ডেলিভারি: এই পণ্যটি গুণগত মান বজায় রাখতে শুধু মাত্র ঢাকা শহরের ভিতরে আইস ব্যাগ এর মাধ্যমে ডেলিভারি করা হয়।
Discount
Price: ৳ 960
MRP:
৳
980
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
A mixture of 75% insulin lispro protamine and 25% insulin lispro injection (rDNA origin) is indicated for the treatment of diabetic patients to control hyperglycemia.
A mixture of 50% insulin lispro suspension and 50% insulin lispro injection (rDNA origin) is also indicated in the treatment of diabetic patients to control hyperglycemia.
Pharmacology
Insulin lispro is a short-acting biosynthetic human insulin analog. Insulin lispro protamine is an intermediate-acting hypoglycemic agent; it is a suspension of crystals made by combining insulin lispro and protamine sulfate under the right conditions for crystal formation. They are used together to regulate glucose metabolism.
Dosage & Administration
Combination rapid-onset (faster than regular insulin) and intermediate-acting insulins in fixed doses. The dose regimen varies among patients depending on metabolic needs; typical daily insulin requirements range between 0.5-1 unit/kg
Interaction
Effects may be enhanced by: oral antidiabetics, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAOIs, propoxyphene, salicylates, somatostatin analogs (eg, octreotide ), sulfonamide antibiotics. The effect may be reduced by: corticosteroids, niacin, danazol, diuretics, sympathomimetics, isoniazid, phenothiazine derivatives, somatropin, thyroid hormone, oral contraceptives, and lithium. Signs of hypoglycemia may be masked by beta blockers and clonidine.
Contraindications
Insulin lispro, protamine, and insulin lispro are contraindicated during hypoglycemic episodes and in patients sensitive to insulin lispro or any excipient contained in the formulation.
Side Effects
Hypoglycemia; hypokalemia, edema; itchy; rash, nausea, rash; hypersensitivity reactions; lipoatrophy or lipo hypertrophy with SC Inj.
Pregnancy & Lactation
Animal reproduction studies have not shown a risk to the fetus and there are no adequate and well-controlled studies on pregnant women OR Animal studies that have shown an adverse effect. , but adequate and well-controlled studies in pregnant women have not shown a risk to the fetus in any trimester.
Breast-feeding: Not known if it is distributed in breast milk; compatible with breastfeeding, but breastfeeding women may require dosage adjustment; cautiously recommend
Precautions & Warnings
Kidney or liver failure; pregnancy, lactation; switch from another insulin. Monitor blood sugar, potassium, electrolytes, HbA1c, and lipids. Concomitant diseases, especially infectious diseases.
Storage Conditions
Store in a refrigerator at 28 ° C. Do not freeze. If recently used insulin does not need to be refrigerated, try to keep it in a cool place, away from heat and light. Insulin in use can be stored at room temperature for one month.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.