✔ 100% Authentic Product
👁️ Currently Viewing 1901
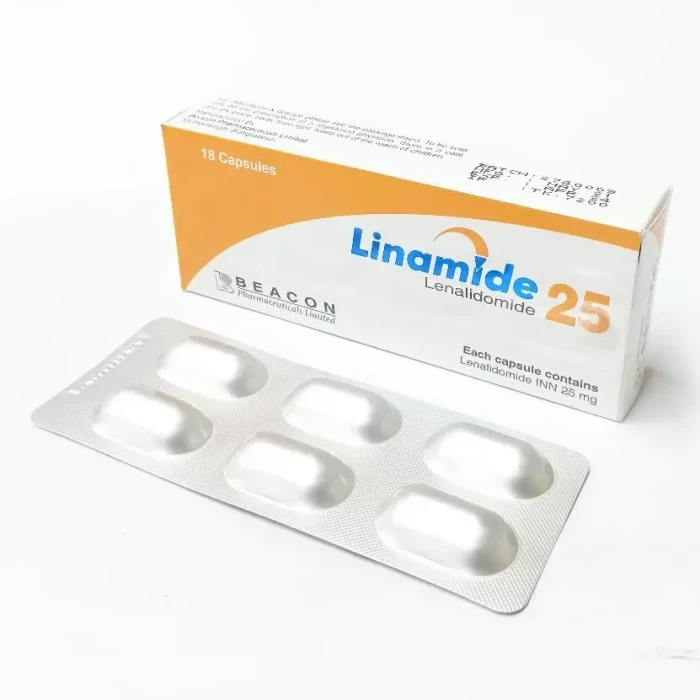
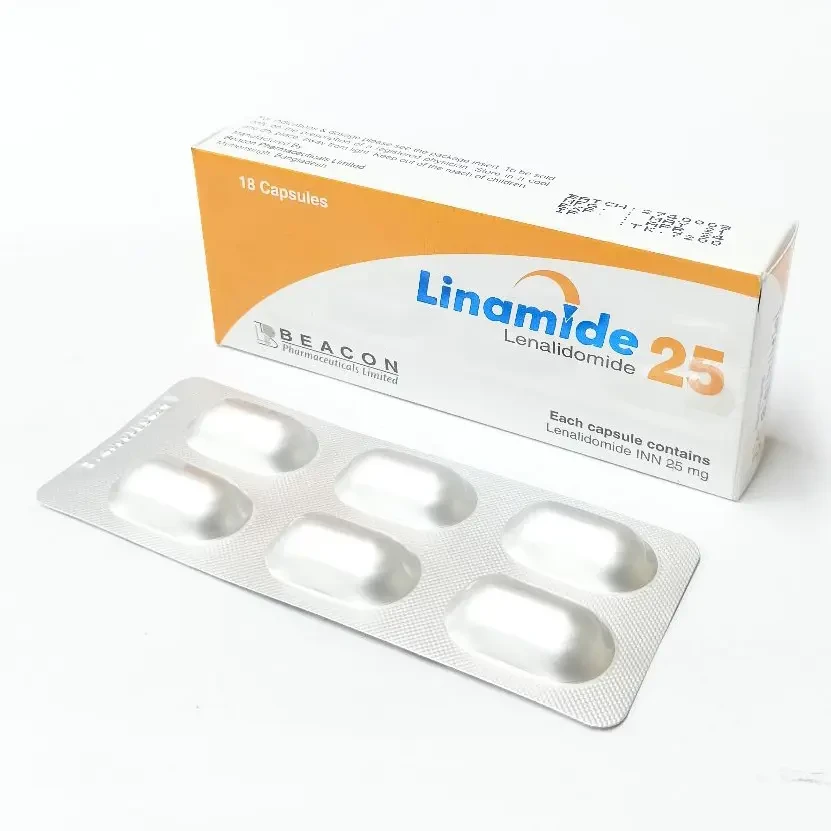
Linamide 25mg 6pcs Capsule
Lenalidomide is used to treat Multiple myeloma, myelodysplastic syndromes, mantle cell lymphoma, and follicular lymphoma.
Discount
Price: ৳ 2,352
MRP:
৳
2400
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
✔️ Multiple Myeloma (MM):
Linamide, in combination with Dexamethasone, is prescribed for adults with MM. It is also used as maintenance therapy after autologous hematopoietic stem cell transplantation (auto-HSCT).
✔️ Myelodysplastic Syndromes (MDS):
Linamide treats adults with transfusion-dependent anemia caused by low- or intermediate-1-risk MDS associated with deletion 5q cytogenetic abnormality, with or without additional cytogenetic changes.
✔️
Mantle Cell Lymphoma (MCL):
Linamide is used to treat adults with relapsed or progressive MCL after two prior therapies, one of which included Bortezomib.
✔️ Follicular Lymphoma (FL):
Linamide, when combined with Rituximab, is used for adults with previously treated FL.
✔️ Marginal Zone Lymphoma (MZL):
Linamide, in combination with Rituximab, is prescribed for adults with previously treated MZL.
✔️ Pharmacology
Linamide (Lenalidomide) is a thalidomide analog with immunomodulatory, antiangiogenic, and anticancer properties. Its effects are mediated through cereblon, a component of a cullin ring E3 ubiquitin ligase complex, leading to ubiquitination and degradation of proteins like Aiolos, Ikaros, and CK1α. This mechanism contributes to its cytotoxic and immunomodulatory effects.
It inhibits tumor cell proliferation and induces apoptosis in MM, MCL, and del(5q) MDS. Linamide boosts T-cell and NK-cell activity, enhancing antibody-dependent cytotoxicity while reducing inflammatory cytokines (e.g., TNF-α, IL-6). Combined with Dexamethasone, it synergistically reduces cell proliferation and enhances apoptosis in MM cells.
✔️ Pharmacokinetics
Linamide is quickly absorbed after oral intake, with peak plasma levels reached within 0.5 to 6 hours. It shows linear pharmacokinetics without drug accumulation at the recommended dose. High-fat meals may reduce their absorption slightly.
About 30% of Linamide binds to plasma proteins. Trace amounts are found in semen up to 24 hours post-administration. Linamide undergoes minimal metabolism. Two minor metabolites, 5-Hydroxy-Lenalidomide and N-Acetyl-Lenalidomide, each constitute less than 5% of circulating drug levels. Primarily eliminated via the kidneys, with ~90% excreted in urine and ~4% in feces within 10 days.
✔️ Common adverse effects include:
- Embryo-fetal toxicity
- Hematologic toxicity (neutropenia, thrombocytopenia)
- Thrombosis (DVT, PE)
- Hepatotoxicity
- Severe cutaneous reactions (e.g., Stevens-Johnson syndrome)
- Secondary malignancies
✔️ Recommended Dosage
- Multiple Myeloma (Combination with Dexamethasone):
25 mg daily on Days 1-21 of a 28-day cycle. For patients >75 years, reduce the Dexamethasone dose. Continue treatment until disease progression or unacceptable side effects. - Maintenance Therapy After Auto-HSCT:
Start with 10 mg daily (Days 1-28 of 28-day cycles) after hematologic recovery. Increase to 15 mg daily after 3 cycles if tolerated. - Myelodysplastic Syndromes:
10 mg daily. Adjust treatment based on clinical response and lab findings. - Mantle Cell Lymphoma:
25 mg daily on Days 1-21 of 28-day cycles. Continue until disease progression or unacceptable toxicity. - Follicular Lymphoma or Marginal Zone Lymphoma:
20 mg daily on Days 1-21 of 28-day cycles for up to 12 cycles, in combination with Rituximab.
Note: Take capsules whole, with water, and at the same time daily, with or without food.
✔️ Interactions
Digoxin:
May increase Digoxin levels. Monitor Digoxin plasma concentrations.
Thrombosis Risk:
Use erythropoietic or estrogen-containing agents cautiously due to increased thrombosis risk.
Warfarin:
Close monitoring of PT/INR is recommended when co-administered.
✔️ Warnings & Precautions
Pregnancy:
Contraindicated due to severe risk of fetal harm. Strict pregnancy prevention programs must be followed.
Hematologic Toxicity:
Can cause significant neutropenia and thrombocytopenia. Regular blood count monitoring is essential.
Thrombosis Risk:
Increased risk of venous and arterial thromboembolic events. Thromboprophylaxis is recommended for at-risk patients.
Severe Hypersensitivity Reactions:
Can cause angioedema, Stevens-Johnson syndrome, or toxic epidermal necrolysis. Discontinue immediately if severe reactions occur.
Hepatotoxicity:
May cause liver failure. Monitor liver enzymes regularly.
Second Primary Malignancies:
Monitor for secondary cancers during treatment.
✔️ Storage:
Store below 30°C in a dry, dark place. Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.