✔ 100% Authentic Product
👁️ Currently Viewing 3087
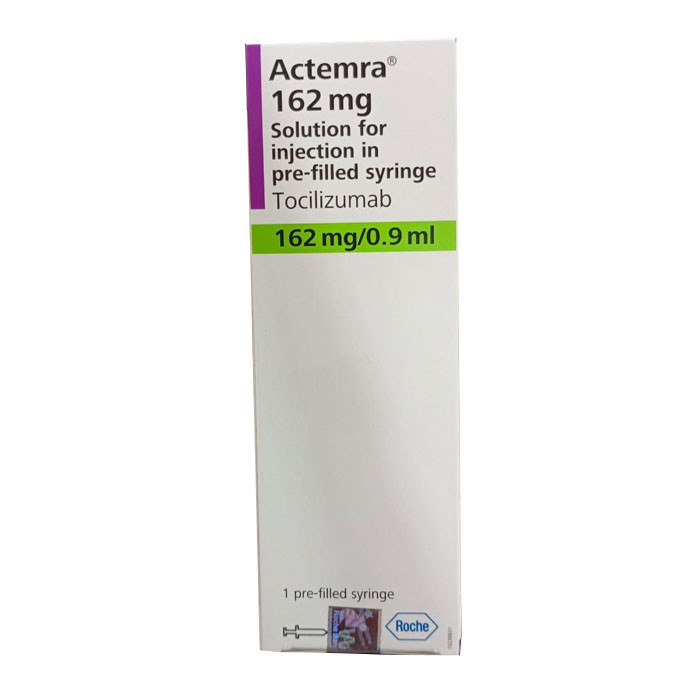
Actemra SC Injection 162mg/0.9ml
Tocilizumab is used in the treatment of ankylosing spondylitis, rheumatoid arthritis, psoriasis, Ulcerative colitis, and Crohn’s disease.
📄Prescription Required
Discount
Price: ৳ 20,204
MRP:
৳
20829
3%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Actemra 162mg/0.9ml SC Injection contains Tocilizumab which belongs to the group of medicines called interleukin-6 (IL-6) receptor inhibitors. It is used to manage adults with moderate to severely active rheumatoid arthritis (an inflammatory disease affecting the joints).
It is also used in children aged 2 years and above to manage active systemic juvenile idiopathic arthritis and polyarticular juvenile idiopathic arthritis (inflammatory disease that causes pain and swelling in one or more joints).
Actemra 162mg/0.9ml SC Injection is also used to manage adults and children aged 2 years and over with severe cytokine release syndrome (a side-effect in patients managed with chimeric antigen receptor (CAR) T-cell therapies used to manage certain types of cancer) and to manage adults with coronavirus disease 2019 (COVID-19), receiving systemic corticosteroids and requiring supplemental oxygen or mechanical ventilation.
Actemra 162mg/0.9ml SC injection is not recommended in patients with active, severe infection, active liver disease, or liver impairment. It should be used with caution in patients with tuberculosis, mild to moderate kidney disease, intestinal ulcers or diverticulitis, and cancer. Consult your doctor before receiving it.
Actemra 162mg/0.9ml SC Injection is not recommended for use in pregnant women, breastfeeding mothers, and children under 2 years of age. Consult your doctor before receiving it.
Safety Advices

Alcohol
UNSAFE
You are recommended to avoid alcohol consumption if you are being treated with Actemra 162mg/0.9ml SC Injection. Alcohol intake, along with Actemra 162mg/0.9ml SC Injection, may cause increased dizziness and risk of stomach bleeding/ulcers.

Pregnancy
CONSULT YOUR DOCTOR
Actemra 162mg/0.9ml SC Injection is not recommended for use in pregnant women unless clearly necessary. Women of childbearing potential must use effective contraception during and up to 3 months after> management. Contact your doctor before receiving Actemra 162mg/0.9ml SC Injection.

Breastfeeding
CONSULT YOUR DOCTOR
Actemra 162mg/0.9ml SC Injection is not recommended for use in breastfeeding mothers as it is not known if it is excreted in the breast milk. Leave a gap of at least 3 months after your last management before starting breastfeeding. Consult your doctor before receiving Actemra 162mg/0.9ml SC Injection.

Driving
UNSAFE
Do not drive or operate any machines if you feel dizzy after receiving an Actemra 162mg/0.9ml SC Injection.

Kidney
CONSULT YOUR DOCTOR
Actemra 162mg/0.9ml SC Injection should be used with caution in patients with mild to moderate kidney disease with no dose adjustment. There is no information available on the use of this medicine in severe kidney disease. Consult your doctor before receiving Actemra 162mg/0.9ml SC Injection.

Liver
CONSULT YOUR DOCTOR
Actemra 162mg/0.9ml SC Injection is not recommended in patients with active liver disease or liver impairment. Consult your doctor before receiving Actemra 162mg/0.9ml SC Injection.
✔️ Uses of Actemra 162mg/0.9ml SC Injection
To manage:
- Moderate to severe active rheumatoid arthritis in adults
- Systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis in children
- Cytokine release syndrome in adults and children
- Coronavirus disease 2019 (COVID-19) in adults
✔️ How does Actemra 162mg/0.9ml SC Injection work?
Actemra 162mg/0.9ml SC Injection works by blocking the activity of a specific protein (cytokine) called interleukin 6, which is involved in inflammatory processes of the body, and blocking it can reduce the pain and swelling in your joints.
✔️ Side Effects of Actemra 162mg/0.9ml SC Injection
Common adverse reactions (≥5%) include upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased ALT, and injection site reactions. In COVID-19 patients, common adverse reactions (≥3%) include constipation, anxiety, diarrhea, insomnia, hypertension, and nausea.
✔️ Quick Suggestions:
- Tocilizumab is given as a drip (intravenous infusion) or as an injection directly into a vein.
- You may be asked for regular blood tests to check blood counts, cholesterol levels, and liver function during the treatment.
- It might make you feel dizzy. If this happens, avoid driving or operating on machinery.
- It makes it hard to fight with an infection. Inform your doctor if you notice fever, cough, or stomach pain.
- Inform your doctor if you are pregnant, planning to become pregnant, or are breastfeeding.
- Do not stop taking medicine without talking to your doctor first.
✔️ Actemra is an interleukin-6 (IL-6) receptor antagonist indicated for the treatment of:
- Rheumatoid Arthritis (RA): For adult patients with moderately to severely active rheumatoid arthritis who have not responded adequately to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
- Polyarticular Juvenile Idiopathic Arthritis (PJIA): For patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis.
- Systemic Juvenile Idiopathic Arthritis (SJIA): For patients aged 2 years and older with active systemic juvenile idiopathic arthritis.
- COVID-19: Emergency use authorization for Actemra in the treatment of COVID-19 in hospitalized adults and pediatric patients (aged 2 years and older) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
- Cytokine Release Syndrome (CRS): For adults and pediatric patients aged 2 years and older with severe or life-threatening cytokine release syndrome induced by chimeric antigen receptor (CAR) T-cell therapy.
- Giant Cell Arteritis (GCA): For adult patients with giant cell arteritis.
- Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): To slow the decline in lung function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD).
✔️ Pharmacology
Tocilizumab is a recombinant humanized monoclonal antibody against the human interleukin-6 (IL-6) receptor, belonging to the immunoglobulin IgG1K subclass. Tocilizumab binds to both soluble and membrane-bound IL-6 receptors, inhibiting IL-6-mediated signaling. IL-6 is a multifunctional pro-inflammatory cytokine produced by various cell types, including T-cells, B-cells, lymphocytes, monocytes, and fibroblasts. It plays a role in several physiological processes such as T-cell activation, immunoglobulin secretion, hepatic acute phase protein synthesis initiation, and stimulation of hematopoietic precursor cell proliferation and differentiation. IL-6 is also produced by synovial and endothelial cells in joints affected by inflammatory conditions like rheumatoid arthritis.
✔️ Dosage & Administration of Actemra 162mg/0.9ml SC Injection
Dosing may need to be interrupted to manage laboratory abnormalities related to dosage, including elevated liver enzymes, neutropenia, and thrombocytopenia.
Rheumatoid Arthritis: Tocilizumab can be used alone or with methotrexate or other non-biologic DMARDs and can be administered as an intravenous infusion.
Recommended Intravenous Dosage:
- Initial dose: 4 mg per kg of body weight every 4 weeks via a 60-minute intravenous infusion.
- Dose increase: Can be increased to 8 mg per kg of body weight every 4 weeks, depending on clinical response.
- Dose reduction: In cases of certain dose-related laboratory changes, reducing the dose from 8 mg/kg to 4 mg/kg is recommended.
- Doses above 800 mg per infusion are not recommended for RA patients.
Polyarticular Juvenile Idiopathic Arthritis: Tocilizumab can be administered intravenously or subcutaneously, alone or with methotrexate.
Recommended Intravenous Dosage:
- Patients under 30 kg: 10 mg/kg every 4 weeks via a 60-minute intravenous infusion.
- Patients 30 kg or more: 8 mg/kg every 4 weeks via a 60-minute intravenous infusion.
Systemic Juvenile Idiopathic Arthritis: Tocilizumab can be administered intravenously or subcutaneously, alone or with methotrexate.
Recommended Intravenous Dosage:
- Patients under 30 kg: 12 mg/kg every 2 weeks via a 60-minute intravenous infusion.
- Patients 30 kg or more: 8 mg/kg every 2 weeks via a 60-minute intravenous infusion.
COVID-19:
Recommended Dosage:
- Patients under 30 kg: 12 mg/kg via a single 60-minute intravenous infusion.
- Patients 30 kg or more: 8 mg/kg via a single 60-minute intravenous infusion.
- If symptoms worsen or do not improve after the first dose, a second infusion may be given at least 8 hours after the initial infusion. The maximum dosage is 800 mg per infusion.
Cytokine Release Syndrome: Administer only via the intravenous route for CRS treatment.
Recommended Dosage:
- Patients under 30 kg: 12 mg/kg via a 60-minute intravenous infusion, alone or with corticosteroids.
- Patients 30 kg or more: 8 mg/kg via a 60-minute intravenous infusion, alone or with corticosteroids.
- Up to 3 additional doses may be given if no clinical improvement is observed after the first dose, with at least 8 hours between doses. Doses above 800 mg per infusion are not recommended.
Giant Cell Arteritis:
Recommended Dosage:
- 6 mg/kg every 4 weeks via a 60-minute intravenous infusion, in combination with a tapering course of glucocorticoids.
- Actemra can be used alone after discontinuation of glucocorticoids.
- Doses above 600 mg per infusion are not recommended for GCA patients.
Systemic Sclerosis-Associated Interstitial Lung Disease:
Recommended Dosage:
- 162 mg once weekly as a subcutaneous injection. Intravenous administration is not approved for SSc-ILD.
Pediatric Use: The safety and effectiveness of Actemra for conditions other than PJIA, SJIA, or CRS have not been established in pediatric patients. The safety and effectiveness of PJIA, SJIA, or CRS in children under 2 years of age have also not been established. Actemra is not authorized for emergency use in COVID-19 treatment for pediatric patients under 2 years old.
Geriatric Use: Use caution when treating elderly patients, as they have a higher incidence of infections.
Hepatic Impairment: The safety and efficacy of Actemra have not been studied in patients with hepatic impairment, including those with positive HBV and HCV serology.
Renal Impairment: No dose adjustment is required for patients with mild or moderate renal impairment. Actemra has not been studied in patients with severe renal impairment.
Administration of Intravenous Formulation:
- For adults with RA, GCA, CRS, PJIA, and SJIA patients weighing 30 kg or more, dilute to 100 mL in 0.9% or 0.45% Sodium Chloride Injection, USP.
- For PJIA, SJIA, and CRS patients weighing less than 30 kg, dilute to 50 mL in 0.9% or 0.45% Sodium Chloride Injection, USP.
- Administer as a single intravenous infusion over 1 hour; do not administer as a bolus or push.
✔️ Interaction
Use caution when co-administering Actemra with CYP3A4 substrate drugs where decreased effectiveness is undesirable, such as oral contraceptives, lovastatin, and atorvastatin. The effect on CYP450 enzyme activity may persist for several weeks after stopping therapy.
✔️ Contraindications
Actemra is contraindicated in patients with known hypersensitivity to Tocilizumab.
✔️ Pregnancy & Lactation
Based on animal studies, Actemra may cause fetal harm. In the case of COVID-19, use during pregnancy should be considered only if the potential benefits outweigh the potential risks for the mother and fetus. Discontinue the drug or nursing, taking into account the drug's importance to the mother. Breastfeeding individuals with COVID-19 should follow clinical guidelines to avoid exposing the infant to COVID-19.
✔️ Precautions & Warnings
- Serious Infections: Do not administer Actemra during an active infection, including localized infections. If a serious infection occurs, interrupt treatment until the infection is controlled.
- Tuberculosis: Evaluate patients for tuberculosis risk factors and test for latent infection before starting Actemra.
- Gastrointestinal Perforation: Use with caution in patients at increased risk.
- Hepatotoxicity: Monitor for signs and symptoms of liver injury. Modify or discontinue Actemra if abnormal liver tests persist or worsen, or if liver disease symptoms develop.
- Laboratory Monitoring: Regular monitoring is recommended due to potential treatment-related changes in neutrophils, platelets, lipids, and liver function tests.
- Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis and death, have occurred.
- Live Vaccines: Avoid use with Actemra.
- Biological DMARDs: Do not use Actemra in combination with other biological DMARDs.
Actemra should not be started in patients with an absolute neutrophil count (ANC) below 2000/mm³, a platelet count below 100,000/mm³, or ALT or AST levels above 1.5 times the upper limit of normal (ULN).
✔️ Storage:
Store at a temperature of 2°C to 8°C in a dry place. Do not freeze.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.