✔ 100% Authentic Product
👁️ Currently Viewing 1808
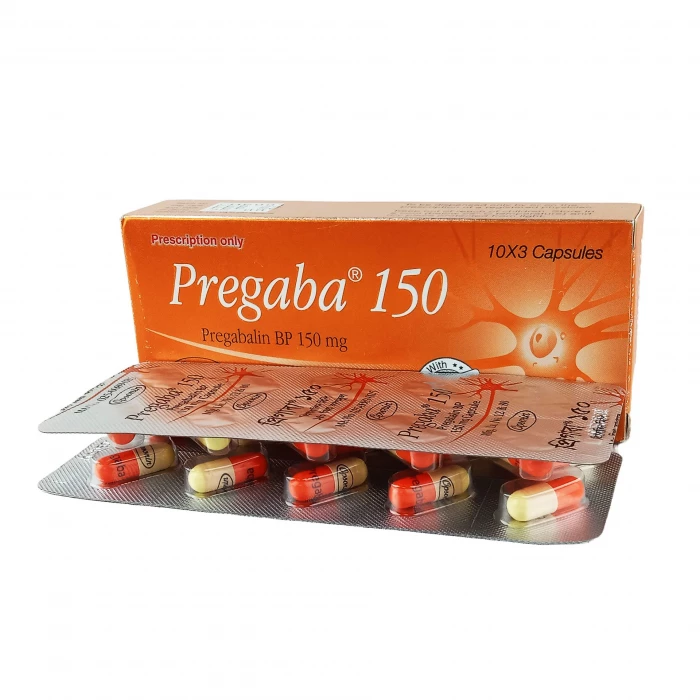
Pregaba 150mg Capsule 10pcs
Type: Caps. Generic Name:Pregabalin INN 75mg & 150mg/capsule, Manufacturer/Distributor: Opsonin
Discount
Price: ৳ 333
MRP:
৳
350
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Pregaba is indicated for:
- Neuropathic pain associated with diabetic peripheral neuropathy (DPN)
- Postherpetic neuralgia (PHN)
- Adjunctive therapy for partial-onset seizures in patients 1 month of age and older
- Fibromyalgia
- Neuropathic pain associated with spinal cord injury
Pregaba CR tablet is indicated for:
- Neuropathic pain associated with diabetic peripheral neuropathy (DPN)
- Postherpetic neuralgia (PHN)
Pharmacology
Pregabalin, a structural derivative of gamma-aminobutyric acid (GABA), binds with high affinity to the alpha2-delta site of voltage-gated calcium channels in the central nervous system. Although its exact mechanism of action is not fully understood, animal studies suggest this binding may contribute to its pain-relieving and seizure-controlling effects.
Dosage
Neuropathic pain associated with diabetic peripheral neuropathy in adults (DPN):
- Maximum dose: 100 mg three times a day (300 mg/day) for patients with creatinine clearance of at least 60 ml/min.
- Initial dose: 50 mg three times a day (150 mg/day), which can be increased to 300 mg/day within a week based on response and tolerability.
- Pregabalin CR: Start with 165 mg once daily, increasing to 330 mg once daily within a week based on response and tolerability. Maximum dose: 330 mg once daily.
Postherpetic neuralgia in adults (PHN):
- Recommended dose: 75 to 150 mg twice daily or 50 to 100 mg three times a day (150 to 300 mg/day) for patients with creatinine clearance of at least 60 ml/min.
- Initial dose: 75 mg twice daily or 50 mg three times a day (150 mg/day), increasing to 300 mg/day within a week based on response and tolerability.
- If insufficient pain relief is experienced with 300 mg/day after 2-4 weeks, and the patient tolerates the drug, the dose may be increased up to 600 mg/day.
- Pregabalin CR: Start with 165 mg once daily, increasing to 330 mg once daily within a week. If insufficient pain relief is experienced with 330 mg once daily after 2-4 weeks, and the patient tolerates the drug, the dose may be increased up to 660 mg once daily.
Fibromyalgia in adults:
- Recommended dose: 300 to 450 mg/day.
- Initial dose: 75 mg twice daily (150 mg/day), increasing to 300 mg/day within a week based on response and tolerability.
- If insufficient benefit is experienced with 300 mg/day, the dose may be increased to 450 mg/day.
Neuropathic pain associated with spinal cord injury in adults:
- Recommended dose range: 150 to 600 mg/day.
- Initial dose: 75 mg twice daily (150 mg/day), increasing to 300 mg/day within a week based on response and tolerability.
- If insufficient pain relief is experienced after 2-3 weeks at 150 mg twice daily, and the patient tolerates the drug, the dose may be increased to 300 mg twice daily.
Conversion from Pregabalin capsules to Pregabalin CR tablets:
- On the day of the switch, take the morning dose of Pregabalin capsule as prescribed and start Pregabalin CR after an evening meal.
Pregabalin tablet to Pregabalin CR capsule dose conversion:
- 75 mg/day: 82.5 mg/day
- 150 mg/day: 165 mg/day
- 225 mg/day: 247.5 mg/day
- 300 mg/day: 330 mg/day
- 450 mg/day: 495 mg/day
- 600 mg/day: 660 mg/day
Administration
Pregabalin is taken orally, with or without food. Pregabalin CR tablets should be taken after an evening meal, swallowed whole, and not split, crushed, or chewed. If a dose is missed, it should be taken as soon as remembered with food, either before bedtime or with the next meal.
Interactions
Drug Interactions: Pregaba is unlikely to be involved in significant pharmacokinetic drug interactions.
Contraindications: Pregabalin is contraindicated in patients with known hypersensitivity to Pregabalin or any of its components.
Side Effects
Common side effects in adults:
- Dizziness, somnolence, dry mouth, edema, blurred vision, weight gain, difficulty with concentration.
Common side effects in pediatric patients:
- Increased weight and appetite.
Pregnancy & Lactation
- There are no adequate studies in pregnant women. Pregnant women should be advised of potential fetal risk.
- Small amounts of pregabalin are detected in the milk of lactating women. Due to potential tumorigenicity risk, breastfeeding is not recommended during treatment with pregabalin.
Precautions & Warnings
- Angioedema (swelling of the throat, head, neck) can occur and may require emergency treatment. Discontinue immediately if this occurs.
- Hypersensitivity reactions (hives, dyspnea, wheezing) require immediate discontinuation.
- Antiepileptic drugs, including Pregaba, may increase the risk of suicidal thoughts or behavior.
- Respiratory depression may occur when used with CNS depressants or in patients with underlying respiratory impairment.
- Pregaba may cause dizziness, somnolence, and impair the ability to drive or operate machinery.
- Rapid discontinuation may increase seizure frequency or cause adverse reactions; taper off gradually over at least one week.
- Pregaba may cause peripheral edema. Caution when coadministering with thiazolidinedione antidiabetic agents.
Use in Special Populations
- Safety and effectiveness in pediatric patients have not been established for neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, neuropathic pain associated with spinal cord injury, and fibromyalgia.
- For partial onset seizures, safety and effectiveness in pediatric patients below the age of 1 month have not been established.
- The safety and effectiveness of Pregaba extended-release tablets in pediatric patients have not been established.
Overdose Effects
Symptoms of overdose include reduced consciousness, depression/anxiety, confusion, agitation, restlessness, seizures, and heart block. There is no specific antidote; supportive care, including monitoring of vital signs and clinical status, is recommended.
Therapeutic Class
- Adjunct anti-epileptic drugs
- Primary anti-epileptic drugs
Storage Conditions
Store in a cool, dry place below 30°C, protected from light and moisture. Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.