✔ 100% Authentic Product
👁️ Currently Viewing 17352
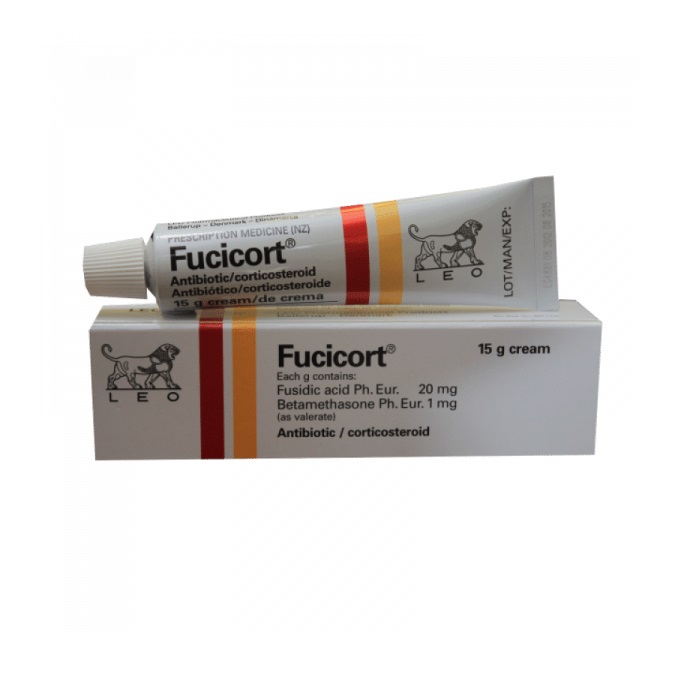
Generic Name: Betamethasone + Fusidic acid
Company Name: LEO Pharma.made in denmark
Discount
Price: ৳ 642
MRP:
৳
690
7%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Description
Each gram contains Fusidic acid 20 mg, Betamethasone as valerate 1 mg.
Preserved with chlorocresol.
Excipients/Inactive Ingredients: macrogol cetostearyl ether, cetostearyl alcohol, chlorocresol, liquid paraffin, sodium dihydrogen phosphate, white soft paraffin, sodium hydroxide (for pH adjustment), all-rac-a-tocopherol, purified water.
Action
ATC Code: D07CC01.
Pharmacology: Fucicort combines the potent topical antibacterial action of fusidic acid with the anti-inflammatory and antipruritic effects of betamethasone. Betamethasone valerate is a topical steroid with rapid effect in inflammatory dermatoses.
Even refractory conditions can often be treated successfully. When applied topically, fusidic acid is effective against Staphylococci, Streptococci, Corynebacteria, Neisseria, and certain Clostridia and Bacteroides. The antibacterial activity of fusidic acid is not diminished in the presence of betamethasone.
Pharmacodynamics: Fucicort Cream combines the potent topical antibacterial action of fusidic acid with the anti-inflammatory and antipruritic effects of betamethasone valerate.
Fusidic acid and its salts exhibit fat and water solubility properties with strong surface activity and show the unusual ability to penetrate intact skin. Concentrations of 0.03 - 0.12 mcg/ml inhibit nearly all strains of Staphylococcus aureus. Topical Fucidin is also active against Streptococci, Corynebacteria, Neisseria, and certain Clostridia.
Betamethasone valerate is a potent topical corticosteroid rapidly effective in those inflammatory dermatoses which normally respond to this form of therapy.
Pharmacokinetics: There are no data that define the pharmacokinetics of Fucicort Cream, following topical administration in man.
However, in vitro studies show that fusidic acid can penetrate intact human skin. The degree of penetration depends on factors such as the duration of exposure to fusidic acid and the condition of the skin. Fusidic acid is excreted mainly in the bile with little excreted in the urine.
Betamethasone is absorbed following topical administration. The degree of absorption is dependent on various factors including skin condition and the site of application. Betamethasone is metabolized largely in the liver but also to a limited extent in the kidneys, and the inactive metabolites are excreted with the urine.
Toxicology: Preclinical safety data: Studies of corticosteroids in animals have shown reproductive toxicity (e.g. cleft palate, skeletal malformations, low birth weight).
Indications/Uses
Fucicort is indicated in inflammatory dermatoses where bacterial infection is present or likely to occur. Inflammatory dermatoses include atopic eczema, discoid eczema, stasis eczema, seborrhoeic, dermatitis, contact dermatitis, lichen simplex chronicus, psoriasis, discoid lupus erythematosus.
Dosage/Direction for Use
Uncovered lesions: 2-3 daily applications.
Covered lesions: Less frequent applications may be adequate.
Overdosage
For topically applied fusidic acid, no information concerning potential symptoms and signs due to overdose administration is available. Cushing's syndrome and adrenocortical insufficiency may develop the following topical application of corticosteroids in large amounts and for more than three weeks.
Systemic consequences of an overdose of active substances after accidental oral intake are unlikely to occur. The amount of fusidic acid in one tube of Fucicort does not exceed the oral daily dose of systemic treatment. A single oral overdose of corticosteroids is rarely a clinical problem.
Contraindications
Hypersensitivity to fusidic acid/sodium fusidate, betamethasone valerate, or any of the Excipients under Description.
Due to the content of corticosteroid, Fucicort is contraindicated in the following conditions: Systemic fungal infections.
Primary skin infections are caused by fungi, viruses, es, or bacteria, either untreated or uncontrolled by appropriate treatment (see Precautions).
Skin manifestations in relation to tuberculosis, either untreated or uncontrolled by appropriate therapy.
Perioral dermatitis and rosacea.
Special Precautions
Long-term continuous topical therapy with Fucicort should be avoided.
Depending on the application site, possible systemic absorption of betamethasone valerate should always be considered during treatment with Fucicort.
Due to the content of corticosteroids, Fucicort should be used with care near the eyes. Avoid getting Fucicort into the eyes (see Adverse Reactions).
Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur following systemic absorption of topical corticosteroids.
Fucicort should be used with care in children as pediatric patients may demonstrate greater susceptibility to topical corticosteroids-induced HPA axis suppression and Cushing's syndrome than adult patients. Avoid large amounts, occlusion, and prolonged treatment (see ADVERSE REACTIONS).
Atrophic changes may occur on the face, and to a lesser degree in other parts of the body, after prolonged treatment with potent topical steroids.
Glaucoma might result if the preparation enters the eye.
Raised intra-ocular pressure and glaucoma may also occur after topical use of corticosteroids near the eyes, particularly with prolonged use and in patients predisposed to developing glaucoma.
Bacterial resistance has been reported to occur with the topical use of fusidic acid. As with all antibiotics, extended or recurrent use of fusidic acid may increase the risk of developing antibiotic resistance. Limiting therapy with topical fusidic acid and betamethasone valerate to no more than 14 days at a time will minimize the risk of developing resistance.
This also prevents the risk that the immunosuppressive action of corticosteroids might mask any potential symptoms of infections due to antibiotic-resistant bacteria.
Due to the content of corticosteroids having immunosuppressant effects, Fucicort may be associated with increased susceptibility to infection, aggravation of existing infection, and activation of latent infection. It is advised to switch to systemic treatment if the infection cannot be controlled with topical treatment (see Contraindications).
Fucicort cream contains cetostearyl alcohol and chlorocresol as excipients. Cetostearyl alcohol may cause local skin reactions (e.g. contact dermatitis) and chlorocresol may cause allergic reactions.
Contact with open wounds and mucous membranes should be avoided.
Effects on the ability to drive and use machines: Fucicort has no or negligible influence on the ability to drive or to use machines.
Use In Pregnancy & Lactation
Pregnancy: Fusidic acid: No effects during pregnancy are anticipated since systemic exposure to fusidic acid is negligible and studies in animals have not shown teratogenic effects.
Betamethasone valerate: There is no or limited amount of data on the use of topical betamethasone valerate in pregnant women. Studies in animal shave showed reproductive toxicity (see Pharmacology: Toxicology: Preclinical safety data under Actions).
Fucicort should not be used during pregnancy unless clearly necessary.
Breastfeeding: No effects on the breastfed newborn/infant are anticipated since the systemic exposure of topically applied fusidic acid and betamethasone valerate to a limited area of skin of the breastfeeding woman is negligible.
Fucicort can be used during breastfeeding but it is recommended to avoid applying Fucicort on the breast.
Fertility: There are no clinical studies with Fucicort regarding fertility.
Adverse Reactions
The estimation of the frequency of undesirable effects is based on a pooled analysis of data from clinical studies and spontaneous reporting.
The most frequently reported adverse reaction during treatment is pruritus.
Undesirable effects are listed by MedDRA SOC and the individual undesirable effects are listed starting with the most frequently reported. Within each frequency grouping, adverse reactions are presented in the order of decreasing seriousness.
Very common ≥1/10, Common ≥1/100 and < 1/10, Uncommon ≥1/1,000 and <1/100, Rare ≥1/10,000 and <1/1,000, Very rare <1/10,000, Not known (cannot be estimated from the available data). (See table.)
Systemic undesirable class effects of corticosteroids like betamethasone valerate include adrenal suppression, especially during prolonged topical administration (see Precautions).
Dermatological undesirable class effects of potent corticosteroids include Atrophy, dermatitis (incl. dermatitis contact and dermatitis acneiform), perioral dermatitis, skin striae, telangiectasia, rosacea, erythema, hypertrichosis, hyperhidrosis, and depigmentation.
Ecchymosis may also occur with prolonged use of topical corticosteroids.
Class effects for corticosteroids have been uncommonly reported for Fucicort as described in the previously mentioned frequency table.
Pediatric population: The observed safety profile is similar in children and adults.
Drug Interactions
No interaction studies have been performed. Interactions with systemically administered medicinal products are considered minimal.
Storage
- Do not store above 30°C.
- Shelf life: 3 years.
- After first opening of container: 3 months.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.