✔ 100% Authentic Product
👁️ Currently Viewing 7399
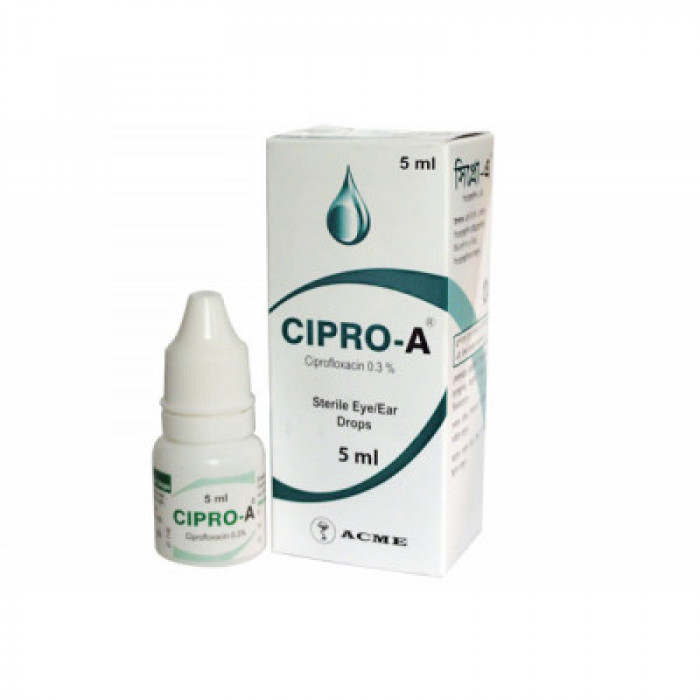
Cipro-A 0.3% Ear/Eye Drops 5ml
Cipro-A 0.3% EYE/EAR Drops contain ciprofloxacin, which is an antibiotic used to treat bacterial infections in the eye or ear, such as corneal ulcers, chronic Suppurative otitis media, external otitis, malignant otitis externa, etc. It is also used to treat inflammatory ear infections in adults and children above 1 year.
Children below 12 months (1 year) of age, including newborns, should not use Cipro-A 0.3% Drops without a doctor's advice. The most common side effects are redness, itching, irritation, pain, red eye, and double vision. If any of these symptoms worsen, it's important to consult a doctor immediately.
However, prolonged use of Cipro-A 0.3% Drops can led to the growth of other non-susceptible organisms. It's important to use it only for the duration your doctor prescribes. Pregnant and breastfeeding women should use it with caution and only if the benefits outweigh the risks.
Discount
Price: ৳ 44
MRP:
৳
46.15
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Eye: This combination eye drop is indicated for the treatment of steroid-responsive inflammatory ocular conditions where bacterial infections or risk of bacterial infections co-exist. The use of a combination drug with an anti-infective component is indicated where the risk of infection is high or where is an expectation that potentially dangerous numbers of bacteria will be present in the eye. The combination can also be used for post-operative inflammation and any other ocular inflammation associated with infection.
Ear: It is indicated for the treatment of ear infections accompanied by inflammation such as otitis externa, otitis media, chronic suppurative otitis media, etc. The combination can also be used for post-operative inflammation of the ear
Frequently reported adverse reactions are transient ocular burning or discomfort. Other reported reactions include stinging, redness, itching, photophobia, conjunctivitis/ keratitis, Periocular/ facial edema, foreign body sensation, blurred vision, tearing, dryness, and eye pain. Elevation of IOP with development of glaucoma, and delayed wound healing may rarely occur.
Cipro-A 0.3% Eye/Ear Drops is an antibiotic, used in the treatment of bacterial eye/ear infections. It relieves the symptoms of the infection by stopping the further growth of the causative microorganisms.
Safety Advices

Alcohol
CAUTION
It is not known if alcohol affects Cipro-A 0.3% Drops. However, it is advisable to limit or avoid alcohol as a precautionary measure.

Pregnancy
CONSULT YOUR DOCTOR
Cipro-A 0.3% Drops should be used during pregnancy only if the potential benefit justifies the potential risk to the developing baby. Consult your doctor before using Cipro-A 0.3% Drops.

Breastfeeding
CONSULT YOUR DOCTOR
Cipro-A 0.3% Drops should be used with caution in breastfeeding mothers due to the lack of safety data in topical use. Consult your doctor before using Cipro-A 0.3% Drops.

Driving
CAUTION
Do not drive or use machinery after using Cipro-A 0.30% Drops as the medicine could temporarily blur or affect your vision

KIdney
CAUTION
Limited information is available about the usage of Cipro-A 0.30% Drops in patients with kidney disease. Please consult your physician.

Liver
CAUTION
Limited information is available about the usage of Cipro-A 0.30% Drops in patients with liver disease. Please consult your physician.
✔️ Uses of Cipro-A 0.3% Drops
- Treatment of Bacterial eye/ear infections
✔️ How does Cipro-A 0.30% Drops work?
Cipro-A 0.30% Drops contains ciprofloxacin, which is an antibiotic with bactericidal activity. It works by inhibiting the activity of the enzyme DNA gyrase in bacteria, which is essential for bacterial DNA synthesis. By doing so, ciprofloxacin prevents the bacteria from replicating and spreading, ultimately leading to their death.
This mechanism of action makes ciprofloxacin an effective treatment for a range of bacterial infections, including external ocular infections of the eye, chronic suppurative otitis media, external otitis, malignant otitis externa, and perioperatively in chronic otitis media. However, it is important to note that ciprofloxacin should only be used to treat bacterial infections and is not effective against viral infections.
✔️ Side Effects of Cipro-A 0.30% Drops
- Eye discomfort
- Ocular hyperemia
- Taste change
- Damage to the eye surface (cornea)
- Sensitivity to light
- Nausea
- Damage to the eye
- Inflammation
- Double vision
- Decreased eye sensation
- Tired eyes
- Blurred vision
- Swelling of the eye and eyelids
- Pain
- Dry eye
- Itchiness
- Eyelids scales
- Eyelid redness
- Watery eyes
- Red eyes
- Headache
- Dizziness
- Ear pain
- Inflammation inside the nose
✔️ Quick Suggestions:
- To maintain healthy eyes, it is recommended to get adequate sleep, wash your eyes with clean water several times a day, and take care of your overall health with a balanced diet, regular exercise, and rest.
- It's important to regularly wash your hands and avoid rubbing your eyes. If you wear makeup, do not share it with others.
- When using contact lenses, clean and replace them frequently, and do not share them with others.
- Limit your screen time and blink frequently to keep your eyes hydrated. If you have been prescribed Cipro-A 0.30% Eye/Ear Drops for a bacterial eye/ear infection, it's crucial to take the full course of treatment and not skip any doses.
- Avoid contaminating the medication by not touching the tip to any surface or your eye/ear.
- Continue to use the medication for 48 hours after symptoms have cleared. If symptoms don't improve or get worse, speak to your doctor.
- If using the drops in the eye, wait for at least 5-10 minutes before applying another medication to avoid dilution.
- Do not wear contact lenses while using the drops, and be cautious if you experience temporary vision blurring after using them.
✔️ Indication of Cipro-A 0.30% Drops
- Treats corneal ulcers and superficial eye infections caused by bacteria, namely Pseudomonas aeruginosa and other gram-negative bacteria that are resistant to standard therapies.
- Treats inflammatory ear infections such as chronic suppurative otitis media, external otitis, malignant otitis externa, and chronic otitis media perioperatively in adults and children over the age of one year.
✔️ Pharmacology
Ciprofloxacin is indeed a broad-spectrum antibacterial drug that is administered intravenously in some cases, although it can also be administered orally or topically in the form of eye drops or ear drops. As you mentioned, the bactericidal effect of ciprofloxacin is due to its ability to suppress the activity of two key enzymes in bacteria, topoisomerase II (DNA gyrase) and topoisomerase IV.
Topoisomerases are enzymes that play a critical role in bacterial DNA replication, transcription, repair, and recombination. By inhibiting the activity of these enzymes, ciprofloxacin prevents the bacteria from properly replicating their DNA and carrying out other essential functions necessary for their survival and growth.
Because ciprofloxacin targets multiple aspects of bacterial DNA metabolism, it is effective against a wide range of bacterial species, including both gram-negative and gram-positive bacteria. However, it is important to note that ciprofloxacin is not effective against all types of bacteria, and some strains may be resistant to the drug. Additionally, as with any antibiotic, it is important to use ciprofloxacin judiciously to minimize the development of antibiotic resistance and potential side effects.
✔️ Dosage & Administrations of Cipro-A 0.30% Drops
- To treat corneal ulcers, the recommended dosage is to apply two drops into the affected eye every 15 minutes for the first six hours, followed by two drops every 30 minutes for the remainder of the first day. On the second day, the dosage should be two drops hourly, and from the third through the fourteenth day, two drops should be placed in the affected eye every four hours. If corneal re-epithelization has not occurred, treatment may continue after 14 days.
- For bacterial conjunctivitis, the recommended dosage is to instill one or two drops into the conjunctival sac(s) every two hours while awake for the first two days, and then one or two drops every four hours while awake for the next five days.
- To treat ear infections, two to three drops should be administered every two to three hours initially, with a reduction in frequency as the infection is controlled. The treatment should be continued for at least seven days.
- It is important to follow the instructions provided by your physician when using Cipro-A 0.30% Drops. This medication is intended for external use only, and you should wash your hands before applying it to the affected area. If you are using this medication in the eye, you should not wear contact lenses while using it.
- Your doctor will determine the appropriate dosage and duration of treatment based on your age and the severity of your condition. It is important to use this medication for the full duration of treatment as prescribed, even if your symptoms improve before completing the treatment course. If you have any questions or concerns about using Cipro-A 0.30% Drops, be sure to consult with your doctor or healthcare provider.
✔️ Interaction
Cipro-A 0.30% Drops have various interactions with other drugs. It may increase the plasma concentrations of CYP1A2 substrates such as clozapine, ropinirole, and theophylline. It can enhance the effect of oral anticoagulants like warfarin and glibenclamide, and increase the toxicity of methotrexate. The plasma concentrations may be increased by probenecid.
The medication's absorption is reduced with oral multivitamins and mineral supplements containing divalent or trivalent cations like iron, zinc, and calcium, and antacids containing aluminum, calcium, or magnesium. Concomitant use with class IA antiarrhythmics like quinidine, and procainamide, and class III antiarrhythmics like amiodarone, sotalol, tricyclic antidepressants, macrolides, and antipsychotics may result in additive effects on QT interval prolongation.
The concurrent use of Cipro-A 0.30% Drops with corticosteroids may increase the risk of severe tendon disorders. There is an increased risk of central nervous system stimulation when using nonsteroidal anti-inflammatory drugs. The serum concentrations of phenytoin may be altered. Additionally, there is a potentially fatal risk of marked elevation in serum levels of tizanidine, which is associated with a potentiated hypotensive and sedative effect.
✔️ Contraindications
Hypersensitivity to the quinolone antibacterial group or any of the formulation's components.
✔️ Pregnancy & Lactation
If the potential benefits outweigh the potential risk during pregnancy, don't use it. It is unknown whether topical ophthalmic administration causes excretion in human milk. Nursing mothers should be treated with caution.
✔️ Precautions & Warnings
- If you are allergic to any of the components of Cipro-A 0.30% Drops, you should not use this medication. Before using this medication, inform your doctor if you have any pre-existing vision problems, severe eye pain, glaucoma (high blood pressure in the eye), eye damage, previous eye surgery, hearing problems (cytotoxicity), or a perforated eardrum and chronic otitis media.
- If you are pregnant, planning to become pregnant, or are breastfeeding, you should inform your doctor before using this medication. Your doctor will weigh the benefits and potential risks of using Cipro-A 0.30% Drops during pregnancy or breastfeeding.
- If your infection symptoms persist or worsen after two weeks of treatment, contact your doctor. They may need to re-evaluate your condition and adjust your treatment plan accordingly.
- Cipro-A 0.30% Drops are generally not recommended for use in children below 1 year of age (12 months) with a doctor’s advice. Consult your doctor before using Cipro-A 0.30% Drops.
✔️ Storage Conditions
Store below 30° C in a cold, dry, and light-protected location. Keep out of children's reach. Because touching the dropper tip to surfaces can contaminate the solution, avoid doing so. After 30 days from the date of initial opening, do not use.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.