✔ 100% Authentic Product
👁️ Currently Viewing 1868
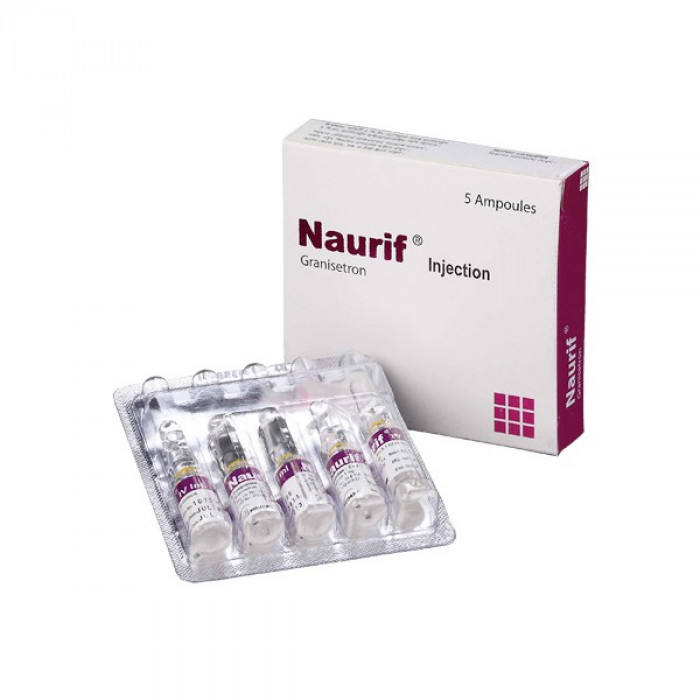
Naurif 1mg Tablet 10pcs
Tablet Manufacturer/Distributor: Square Pharmaceuticals Ltd. Generic Name: Granisetron 1 mg Tablet
Discount
Price: ৳ 268
MRP:
৳
281.9
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
Granisetron Tablet is demonstrated for Queasiness and spewing related with starting and rehash course of emetogenic cancer treatment, counting tall dosage of cisplatin. Queasiness and heaving related with radiation, counting add up to body light and fractionated stomach radiation.
Granisetron Infusion is shown for: The avoidance of sickness and vomitng related with beginning and rehash courses of emetogenic cancer chemotherapy, treatment counting tall measurements cisplatin. The anticipation and treatment of post agent queasiness and heaving.
Pharmacology
Granisetron may be a exceedingly particular 5-HT3 receptor enemy with small or no partiality for other serotonin receptors. It squares serotonin peripherally on vagal nerve terminals and centrally within the chemoreceptor trigger zone.
Serotonin receptors of the 5-HT3 sort are found incidentally on vagal nerve terminals and centrally within the chemoreceptor trigger zone of the range postrema. Amid chemotherapy that actuates spewing, mucosal enterochromaffin cells discharge serotonin, which invigorates 5-HT3 receptors. This brings out vagal afferent release, actuating spewing. Creature ponders illustrate that, in official to 5-HT3 receptors, granisetron squares serotonin incitement and consequent heaving after emetogenic stimuli such as cisplatin. Within the ferret creature show, a single granisetron infusion avoided spewing due to high-dose cisplatin or captured heaving inside 5 to 30 seconds.
In most human considers, granisetron has had small impact on blood weight, heart rate or ECG. No prove of an impact on plasma prolactin or aldosterone concentrations has been found in other ponders.
Dosage & Administration
Tablet-
Emetogenic Chemotherapy: The suggested grown-up dose of verbal Granisetron is 2 mg once day by day or 1 mg twice day by day. Within the 2 mg once-daily regimen, two 1 mg tablets is given up to 1 hour some time recently chemotherapy. Within the 1 mg twice-daily regimen, the primary 1 mg tablet is given up to 1 hour before chemotherapy, and the moment tablet is given 12 hours after the primary. Either regimen is managed as it were on the day(s) chemotherapy is given. Proceeded treatment, whereas not on chemotherapy, has not been found to be useful.
Injection-
Chemotherapy Initiated Sickness and Vomiting:
Adults: The suggested measurement for Granisetron Infusion is 10 mcg/kg managed intravenously inside 30 minutes some time recently start of chemotherapy, and as it were on the day(s) chemotherapy is given. Granisetron Infusion may be managed intravenously either undiluted over 30 seconds, or weakened with 0.9% Sodium Chloride or 5% Dextrose and imbued over 5 minutes. As a common safeguard, Granisetron Infusion ought to not be blended in arrangement with other drugs.
Pediatric Patients: The prescribed dosage in pediatric patients 2 to 16 a long time o f age is 10 mcg/kg. Pediatric patients beneath 2 a long time o f age have not beenstudied.
Geriatric Patients, Renal Disappointment Patients or Hepatically Impaired Patients: No dose alteration is required.
Treatment of Postoperative Queasiness and Vomiting:
Adults: The prescribed measurement for anticipation of postoperative sickness and spewing could, be a single dosage of 1 mg of Granisetron ought to be weakened to 5 ml andadministered as a moderate intravenous infusion (over 30 seconds). Organization ought to be completed earlier to acceptance of anesthesia. The suggested measurement for the treatment of queasiness and spewing after surgery is 1 mg of Granisetron undiluted, managed intravenously over 30 seconds.
Pediatric Patients: Security and adequacy of Granisetron Infusion have not been built up in pediatric patients for the avoidance or treatment of post agent queasiness or vomiting.
Geriatricpatients, Renal Disappointment Patients or Hepatically Disabled Patients: No dose alteration is required.
Interaction
Actuated digestion system with phenobarbital. Chance of serotonin disorder with other serotonergic specialists e.g. SSRIs, and serotonin and norepinephrine reuptake inhibitors (SNRIs). Modified clearance with CYP chemical inducers or inhibitors. Concomitant utilize with drugs known to draw out QT interim may result in clinical results.
Contraindications
Granisetron is contraindicated in patients with known extreme touchiness to granisetro
Side Effects
Cerebral pain, a sleeping disorder, obstruction, loose bowels, lifted hepatic transaminases; QT prolongation; bradycardia, palpitations, wiped out sinus disorder, chest torment. Application location responses (transdermal): Hasty, torment, erythema, pruritus, aggravation, burn, vesicles, urticaria, discolouration; fix non-adhesion.
Pregnancy & Lactation
Pregnancy Category B. No prove of disabled richness or hurt to the creature hatchling have been found. In any case, this sedate may be utilized in pregnancy as it were on the off chance that clearly required. It isn't known whether granisetron is excreted in human drain. So cautions hould be worked out when granisetron is managed to a nursing mother.
Precautions & Warnings
Quiet with cardiac co-morbidities, on cardiotoxic chemotherapy, and/or with concomitant electrolyte variations from the norm. May veil dynamic ileus and/or gastric distention. Children. Pregnancy and lactation.
Storage Conditions
Store between 15-30°C. Ensure from light.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.