✔ 100% Authentic Product
👁️ Currently Viewing 2627
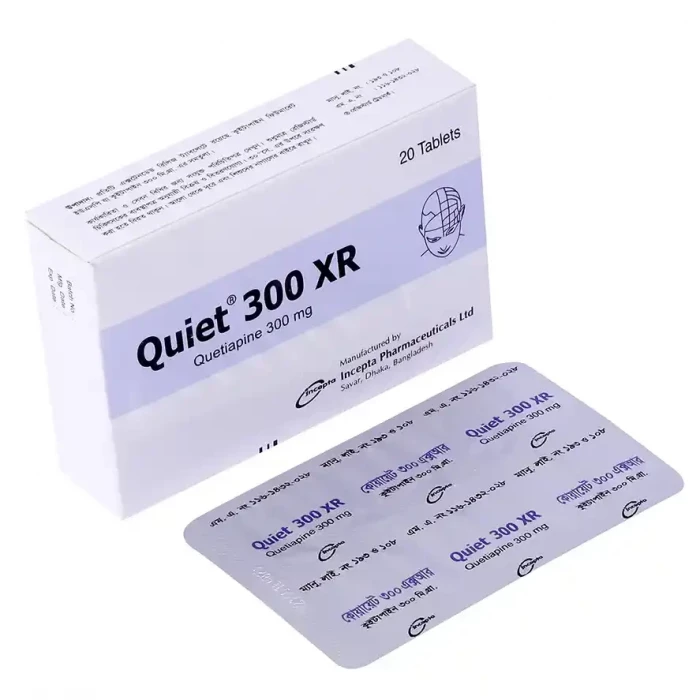
Quiet 300 XR Tablet 10pcs
- Quetiapine is used to treat Schizophrenia, Bipolar disorder, Major depressive disorder
📄Prescription Required
Discount
Price: ৳ 219
MRP:
৳
230
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Quiet XR 300mg Tablet contains Quetiapine, an atypical antipsychotic, used to treat:
- Schizophrenia: A mental disorder that can result in hallucinations or delusions and adversely affects a person’s ability to think and behave.
- Mania: A condition characterized by elevated mood, hyperactivity, and increased energy levels.
- Bipolar Disorders: Mental disorders marked by extreme mood swings, including emotional highs (mania or hypomania) and lows (depression).
Safety Advices

Alcohol
UNSAFE
Avoid consumption of alcohol while taking Quiet XR 300mg Tablet, as it may increase the risk of dizziness or sleepiness.

Pregnancy
UNSAFE
Quiet XR 300mg Tablet is not recommended for use in pregnant women unless specifically directed by your doctor. If you are pregnant, think you may be pregnant or are planning to become pregnant, consult your doctor before taking this medication.

Breastfeeding
UNSAFE
Quiet XR 300mg Tablet is not recommended for use in breastfeeding women. Therefore, it is important to consult your doctor before taking this medication if you are nursing.

Driving
UNSAFE
Do not drive or operate any machinery if you feel dizzy or sleepy after taking Quiet XR 300mg Tablet. This medication can impair your ability to perform tasks that require alertness.

Kidney
CONSULT YOUR DOCTOR
Quiet XR 300mg Tablet should be used with caution in patients experiencing urinary retention (difficulty in completely emptying the bladder). Therefore, it is crucial to consult your doctor before taking this medication if you have kidney issues.

Liver
CONSULT YOUR DOCTOR
Quiet XR 300mg Tablet should be used with caution in patients with liver problems. It is essential to consult your doctor before starting this medication if you have liver conditions.
✔️ Uses of Quiet XR 300mg Tablet
- Schizophrenia
- Bipolar disorder
- Depression
✔️ How does Quiet XR 300mg Tablet work?
It acts by blocking certain receptors (dopamine (D2), serotonin 2A (5HT2A)) in the brain that cause abnormal mood changes and mental problems. As a result, it improves the positive and negative symptoms of schizophrenia and bipolar disorder.
✔️ Side Effects of Quiet XR 300mg Tablet
- Headache
- Dry Mouth
- Constipation
- Dizziness
- Sleepiness
✔️ Quick Suggestions:
- Regular monitoring and consultation with your doctor are essential to manage and adjust the treatment for the best results.
- Inform your doctor of any pre-existing conditions, other medications you are taking, and any side effects you experience.
- Follow your doctor’s instructions carefully regarding the dosage and duration of the medication.
✔️ Indications of Quiet XR 300mg Tablet
Quiet XR 300mg Tablet is used to manage bipolar disorder, schizophrenia, and depression (major depressive disorder). Bipolar disorder (also known as manic depression) is a mental disorder characterized by alternative episodes of mania (sudden unusual extreme sense of happiness) and depression.
✔️ Quetiapine Pharmacology and Mechanism of Action
Chemical Class:
- Quetiapine Fumarate is an atypical psychotropic agent that belongs to the chemical class known as dibenzothiazepine derivatives.
Receptor Antagonism:
- Quetiapine acts as an antagonist at multiple neurotransmitter receptors in the brain, including:
- Serotonin Receptors: 5HT1A and 5HT2
- Dopamine Receptors: D1 and D2
- Histamine Receptors: H1
- Adrenergic Receptors: α1 and α2
Lack of Affinity:
- Quetiapine has negligible affinity for cholinergic muscarinic and benzodiazepine receptors.
Proposed Mechanism of Action:
- The precise mechanism of action for quetiapine is not fully understood. However, its efficacy in treating schizophrenia and other psychoses is thought to be due to the combined antagonism of dopamine D2 and serotonin 5HT2 receptors.
- Dopamine D2 Antagonism: May help reduce the positive symptoms of schizophrenia, such as hallucinations and delusions.
- Serotonin 5HT2 Antagonism: May help alleviate negative symptoms and improve cognitive function.
Side Effects Explained by Receptor Antagonism:
- Somnolence: Likely due to antagonism of histamine H1 receptors.
- Orthostatic Hypotension: Attributed to antagonism of adrenergic α receptors.
✔️ Quetiapine Dosage Guidelines
Schizophrenia
Immediate Release:
- Day 1: 50 mg/day PO divided q12hr
- Days 2-3: Increase daily by 25-50 mg q8-12hr to 300-400 mg by Day 4
- Further Adjustments: Increments of 25-50 mg q12hr at intervals >2 days
- Dosage Range: 150-750 mg/day
Extended Release:
- Day 1: 300 mg/day PO
- Subsequent Increases: Up to 300 mg/day at intervals >1 day
- Maintenance (monotherapy): 400-800 mg/day
- Reinitiation:
- If discontinued >1 week: Retitrate dose
- If discontinued <1 week: Reinitiate at previous maintenance dose
Bipolar I Disorder (Mania)
Immediate Release (Monotherapy or Adjunct to Lithium/Divalproex):
- Day 1: 100 mg/day PO divided q12hr
- Day 2: 200 mg/day PO divided q12hr
- Day 3: 300 mg/day PO divided q12hr
- Day 4: 400 mg/day PO divided q12hr
- Further Adjustments: Up to 800 mg/day by Day 6 in increments <200 mg/day
- Dosage Range: 400-800 mg/day (max 800 mg/day)
Extended Release:
- Day 1: 300 mg PO once daily
- Day 2: 600 mg PO once daily
- Maintenance (Day 3 onward): 400-800 mg/day PO
Bipolar Disorder (Depressive Episodes)
Immediate Release or Extended Release:
- Day 1: 50 mg PO at bedtime
- Day 2: 100 mg PO at bedtime
- Day 3: 200 mg PO at bedtime
- Maintenance (Day 4 onward): 300 mg PO at bedtime
Bipolar I Disorder (Maintenance)
Immediate Release:
- Dosage Range: 400-800 mg/day PO divided q12hr
Extended Release:
- Dosage Range: 400-800 mg/day PO in a single dose
Major Depressive Disorder
Extended Release (Adjunct to Antidepressants):
- Days 1 and 2: 50 mg PO in the evening
- Day 3: May increase to 150 mg PO in the evening
- Dosage Range: 150-300 mg/day
Special Populations
Elderly:
- The slower rate of dose titration and lower daily therapeutic dose are recommended.
Hepatic Impairment:
- Initial dose: 25 mg daily
- Increase in increments of 25-50 mg daily until effective dose according to response and tolerability.
Pediatric Dose
Schizophrenia:
Immediate Release:
- Day 1: 50 mg/day PO divided q12hr
- Days 2-3: Increase daily by 25-50 mg q8-12hr to 300-400 mg by Day 4
- Further Adjustments: Increments of 25-50 mg q12hr at intervals >2 days
- Dosage Range: 150-750 mg/day
Extended Release:
- Day 1: 300 mg/day PO
- Subsequent Increases: Up to 300 mg/day at intervals >1 day
- Maintenance (monotherapy): 400-800 mg/day
- Reinitiation:
- If discontinued >1 week: Retitrate dose
- If discontinued <1 week: Reinitiate at previous maintenance dose
Bipolar I Disorder (Mania):
Immediate Release (Monotherapy or Adjunct to Lithium/Divalproex):
- Day 1: 100 mg/day PO divided q12hr
- Day 2: 200 mg/day PO divided q12hr
- Day 3: 300 mg/day PO divided q12hr
- Day 4: 400 mg/day PO divided q12hr
- Further Adjustments: Up to 800 mg/day by Day 6 in increments <200 mg/day
- Dosage Range: 400-800 mg/day (max 800 mg/day)
Extended Release:
- Day 1: 300 mg PO once daily
- Day 2: 600 mg PO once daily
- Maintenance (Day 3 onward): 400-800 mg/day PO
Bipolar Disorder (Depressive Episodes):
- Day 1: 50 mg PO at bedtime
- Day 2: 100 mg PO at bedtime
- Day 3: 200 mg PO at bedtime
- Maintenance (Day 4 onward): 300 mg PO at bedtime
Bipolar I Disorder (Maintenance):
Immediate Release:
- Dosage Range: 400-800 mg/day PO divided q12hr
Extended Release:
- Dosage Range: 400-800 mg/day PO in a single dose
Administration: This can be taken with or without food. It is advised to take it at the same time each day to maintain consistent medication levels in the body.
- Missed Dose: If you miss a dose, take it as soon as you remember. However, if it is close to the time of your next dose, skip the missed dose and resume your usual dosing schedule.
- Discontinuation: Do not stop taking Quiet XR 300 suddenly without consulting your doctor, as it may worsen your symptoms.
✔️ Drug Interactions and Precautions for Quetiapine
Increased Risk of Drowsiness and Postural Hypotension:
- Alcohol: Consuming alcohol while taking quetiapine can increase the risk of drowsiness and postural hypotension. It is advisable to avoid alcohol to prevent these effects.
Interaction with CYP3A4 Enzyme:
- CYP3A4 Inducers: Medications such as phenytoin and carbamazepine are known to induce the CYP3A4 enzyme. These inducers can decrease the plasma levels of quetiapine, potentially reducing its efficacy.
- Phenytoin and Carbamazepine: May require dosage adjustments of quetiapine to maintain therapeutic levels.
- CYP3A4 Inhibitors: Medications such as ketoconazole and erythromycin inhibit the CYP3A4 enzyme. These inhibitors can increase the plasma levels of quetiapine, potentially increasing the risk of side effects.
- Ketoconazole and Erythromycin: May necessitate a lower dose of quetiapine to avoid toxicity.
Recommendations
- Avoid Alcohol: Patients should be advised to avoid alcohol while on quetiapine therapy due to the risk of increased drowsiness and postural hypotension.
- Monitor Drug Interactions:
- With CYP3A4 Inducers (e.g., Phenytoin, Carbamazepine): Regular monitoring of quetiapine levels and possibly increasing the dose of quetiapine.
- With CYP3A4 Inhibitors (e.g., Ketoconazole, Erythromycin): Monitoring for signs of quetiapine toxicity and possibly reducing the dose of quetiapine.
✔️ Contraindications
Avoid taking Quetiapine if you are allergic to it. Seek immediate medical attention if you notice any symptoms such as skin rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, breathing difficulty, etc.
✔️ Pregnancy & Lactation
The safety and efficacy of Quetiapine during human pregnancy have not been established. Therefore, Quetiapine should only be used during pregnancy if the benefits justify the potential risks and the administered dose and duration of treatment should be as low and as short as possible. The degree to which Quetiapine is excreted into human milk is unknown. Women who are breast-feeding should therefore be advised to avoid breast-feeding while taking Quetiapine.
✔️ Precautions & Warnings
- Neuroleptic Malignant Syndrome (NMS): Discontinue immediately and seek medical help if you experience symptoms like fever, muscle rigidity, and altered consciousness or seizures.
- Orthostatic Hypotension: Initially, this medicine may cause a sudden drop in blood pressure when changing positions. Rise slowly if you have been sitting or lying down to prevent dizziness.
- Driving and Machinery: Causes dizziness and sleepiness; avoid driving or operating heavy machinery until you know how this medicine affects you.
- Weight Gain: May increase weight; adopt a healthy diet and exercise regularly to manage this side effect.
- Diabetes Risk: May increase the risk of developing diabetes; monitor blood glucose levels regularly.
- Mood Changes: Report any unusual changes in mood or behavior, new or worsening depression, or suicidal thoughts to your doctor.
✔️ Storage Conditions:
- Store in a cool, dry place away from light.
- Keep out of reach of children.
- Do not use it after the expiry date stated on the blister pack and carton. The expiry date refers to the last day of that month.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.