✔ 100% Authentic Product
👁️ Currently Viewing 1694
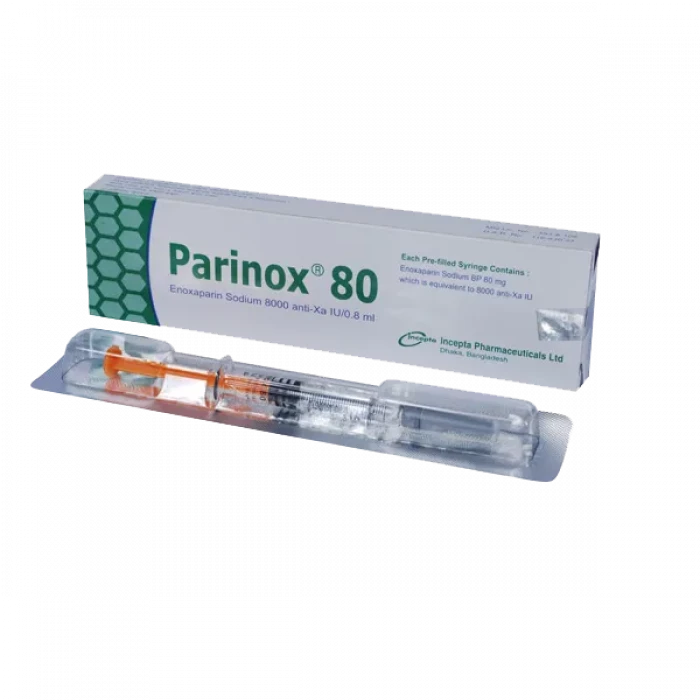
Parinox 80 Injection
Injection, Generic Name: Enoxaparin Sodium , Manufacturer: Incepta Pharmaceuticals Ltd.
Discount
Price: ৳ 618
MRP:
৳
650
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Indications of Parinox 80 SC Injection
Enoxaparin represents deep vein thrombosis, whether it is
▪ embolism of the palace.
▪ I will treat an unstable angorne myocardial infarction and non-wave and administered with aspirin simultaneously.
▪ Prevent thrombosis in extrapolation during blood dialysis.
▪ Prevention of venous thrombosis (prevention of blood flow veins formation), especially those that can be related to orthopedic or general surgical surgery.
▪ Prevention of venous thrombus diseases due to acute diseases due to acute illnesses, including cardiac dysfunction, respiratory failure, serious infections and rheumatoid diseases.
Pharmacology of Parinox 80 SC Injection
Enoxaparin sodium is a low molecular weight heparin with high antiXa activity and low antilles or antithrombin activity. At doses required for various indications, enoxaparin sodium did not increase bleeding time. At preventive doses, Enoxaparin Sodium did not cause any noticeable change in Partial Thrombosis Trigger Time (PTT). It does not affect platelet aggregation or the binding of fibrinogen to platelets. Enoxaparin sodium is metabolised mainly in the liver.
Dosage & Administration of Parinox 80 SC Injection
Treatment of deep vein thrombosis, with or without pulmonary embolism: Subcutaneously 100 anti-Xa lU/kg twice daily for 10 days or Subcutaneously 150 anti-Xa lU/kq once daily for 10 days. Oral anticoagulant therapy should be initiated when appropriate and Enoxaparin Sodium treatment should be continued until a therapeutic anticoaqulant effect has been achieved.
Treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin: Subcutaneously 100 anti-Xa lU/kg twice daily for 2- 8 days. Should be administered concurrently with oral aspirin (100 to 325 mg once daily). Treatment with Enoxaparin Sodium in these patients should be prescribed fora minimum of 2 days and continued until clinical stabilization.
Prevention of thrombus formation in extra corporeal circulation during hemodialysis: Recommended dose is 100 anti-Xa lU/kg. For patients with a high risk of hemorrhage, the dose should be reduced to 50 anti-Xa lU/kg for double vascular access or 75 anti-Xa lU/kg for single vascular access. During hemodialysis, Enoxaparin Sodium should be introduced into the arterial line of the circuit at the beqinninq of the dialysis session.
Prophylaxis of venous thromboembolic disease in surgical patients:
Patients undergoing general surgery with a moderate risk of thromboembolism (e.g. abdominal surgery): Subcutaneously 2000 anti-Xa IU (0.2 ml) or 4000 anti-Xa IU (0.4 ml) once daily for 7 to 10 days. The first injection should be given 2 hours before the surgical procedure.
Patients undergoing orthopedic surgery with a high risk of thromboembolism: Subcutaneously 4000 anti-Xa IU (0.4 ml) once daily for 7 to 10 days. The first injection should be given 12 hours before the surgical procedure. Longer treatment duration may be appropriate in some patients like continued therapy with 4000 anti-Xa IU once daily for 3 weeks following the initial therapy has been proven to be beneficial in orthopaedic surqery.
Prophylaxis of venous thromboembolic disease in medical patients: Subcutaneously 4000 anti-Xa IU (0.4 ml) once daily for 6- 14 days.
Interaction of Parinox 80 SC Injection
It is recommended that agents that affect hemostasis be discontinued prior to treatment with enoxaparin sodium, unless otherwise indicated. These agents include drugs such as: acetylsalicylic acid (and derivatives), NSAIDs (including ketorolac), ticlopidine, clopidogrel, dextran 40, glucocorticoids, thrombolytics and anticoagulants, substances other antiplatelet agents, including the glycoprotein antagonist llb/llla. If the combination is indicated, it should be used with careful clinical and laboratory monitoring.
Contraindications
Patients with hypersensitivity to enoxaparin sodium, heparin or other low molecular weight heparins. Patients with heavy bleeding and conditions with a high risk of uncontrolled bleeding, including recent hemorrhagic stroke.
Side Effects of Parinox 80 SC Injection
Haemorrhage (bleeding), thrombocytopenia, increase in serum aminotransferases. Painful bluish spots at the injection site to a rash at the injection site. Cases of nerve hematoma with concomitant administration of enoxaparin and spinal / epidural anesthesia or lumbar puncture have resulted in varying degrees of neurological damage.
Pregnancy & Lactation
Pregnancy category B. In humans, there is no evidence that enoxaparin sodium crosses the placental barrier. In the absence of adequate and well-controlled studies in pregnant women, enoxaparin sodium should be used during pregnancy only if clearly needed. Pregnant women with prosthetic mechanical heart valves may have a higher risk of thromboembolism. It is not known whether enoxaparin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse events in the nursing infant, a decision should be made whether to discontinue nursing or to discontinue Enoxaparin, taking into account the importance of Enoxaparin to the mother. and the known benefits of breastfeeding.
Precautions & Warnings
Enoxaparin sodium should be injected subcutaneously for prophylactic and curative treatment and intravascularly during hemodialysis. Do not inject intramuscularly. Enoxaparin sodium should be used with caution in conditions that increase the risk of bleeding, such as poor hemostasis, history of peptic ulcer disease, recent ischemic stroke, severe hypertension without control, diabetic retinopathy, and recent ophthalmic or neurological surgery, concomitant use of drugs that affect hemostasis. It is recommended that platelet counts be measured prior to initiation of treatment and frequently thereafter during treatment.
Storage Conditions
Store in a cool and dry place, protect from light and moisture. Do not store above 25°C. Do not store in a refrigerator or freezer. Keep out of the reach of children
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.