✔ 100% Authentic Product
👁️ Currently Viewing 238
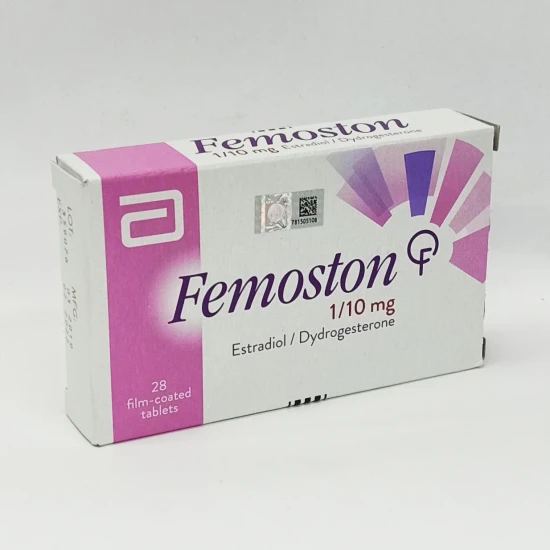
Femoston 1/10mg Tablet
Femoston 1/10 Tablet contains Estradiol and Dydrogesterone, used to treat postmenopausal symptoms and prevent osteoporosis (weak and brittle bones) caused by menopause in women. Estradiol restores the falling levels of estrogen in the body and thus helps to reduce menopause symptoms. Dydrogesterone decreases the overgrowth of the womb lining. Also, Femoston 1/10 Tablet is used as prophylaxis in preventing osteoporosis in postmenopausal women and prevents bone loss and fractures.
Discount
Price: ৳ 16,552
MRP:
৳
16890
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Femoston 1/10 Tablet is used in the treatment and prevention of hot flashes and osteoporosis in postmenopausal women. The detailed uses of Femoston 2/10 Combipack Tablet are as follows:
- Femoston 1/10 Tablet helps relieve hot flashes, night sweats, and mood changes associated with menopause.
- Femoston 1/10 Combipack Tablet helps maintain bone density and reduces the risk of fractures by compensating for decreased estrogen levels after menopause.
- Femoston 1/10 Tablet restores hormone levels in postmenopausal women, improving overall quality of life and preventing complications due to estrogen deficiency.
Femoston 1/10 should be avoided if you are allergic to it or if you have a history of breast cancer, endometrial cancer (cancer of the lining of the womb), unexplained vaginal bleeding, untreated endometrial hyperplasia (thickening of the womb lining), blood clotting disorder, heart attack or angina (chest pain), inherited porphyria (a blood disorder) or liver disease.
✔️ Side Effects | Femoston 1/10
- Pain Or Discomfort In The Breast
- Recurrent Severe Headache
- Dizziness
- Trouble Falling/ Staying Asleep
- Depression
- Increased blood pressure
- Indigestion
✔️ Missed Dose
If a dose is missed, it should be taken as soon as remembered. If more than 12 hours have passed, the missed dose should be skipped and the next dose taken at the usual time. Missing a dose may increase the risk of breakthrough bleeding or spotting.
✔️ Dosage & Administration
Femoston 1/10 and Femoston 2/10 are continuous sequential hormone replacement therapies (HRT) used for managing postmenopausal symptoms. Treatment should always begin with the lowest effective dose and continue for the shortest duration necessary to achieve symptom relief.
Starting Dose
Treatment usually starts with Femoston 1/10. Based on the patient’s clinical response and symptom control, the dose may be adjusted. If symptoms related to estrogen deficiency are not adequately relieved, therapy may be escalated to Femoston 2/10.
Initiating Treatment
Women who are not currently using HRT and are amenorrhoeic, or those switching from continuous combined HRT, may start Femoston on any convenient day.
Women switching from a cyclic or continuous sequential HRT regimen should begin Femoston the day after completing their previous treatment cycle.
Administration Schedule
Each treatment cycle lasts 28 days:
Days 1–14: Take one tablet containing oestradiol daily.
Days 15–28: Take one tablet containing oestradiol and dydrogesterone daily.
At the end of the 28-day cycle, begin the next pack immediately, without any break. Femoston may be taken with or without food.
The blister pack is marked with the days of the week. Tablets should be taken in order—first from the section marked with arrow 1, followed by those marked with arrow 2.
- Femoston Tablet can be taken with or without food as advised by the doctor.
- Follow your doctor’s instructions on the dosage and timing of this medication to ensure safety.
- Swallow the Femoston Tablet as a whole with a glass of water.
- Do not chew, crush, or break it.
✔️ Drug Interaction
No formal interaction studies have been conducted with Femoston. However, the effectiveness of oestrogens and progestogens may be reduced when taken together with medicines that increase the activity of drug-metabolising enzymes, particularly CYP450 enzymes (CYP2B6, CYP3A4, CYP3A5, CYP3A7).
Such medicines include:
Anticonvulsants: phenobarbital, phenytoin, carbamazepine
Anti-infective agents: rifampicin, rifabutin, nevirapine, efavirenz
Although ritonavir and nelfinavir are known as strong CYP3A inhibitors, they may paradoxically increase the metabolism of steroid hormones when used together.
Herbal products containing St. John’s Wort (Hypericum perforatum) may also enhance the breakdown of oestrogens and progestogens via the CYP3A4 pathway.
Increased hormone metabolism may result in reduced therapeutic efficacy and alterations in uterine bleeding patterns.
✔️ Femoston should not be used in patients with:
- Known, suspected, or previous breast cancer
- Oestrogen-dependent malignancies (e.g. endometrial cancer)
- Progestogen-dependent tumours (e.g. meningioma)
- Unexplained vaginal bleeding
- Untreated endometrial hyperplasia
- Current or past venous thromboembolism (deep vein thrombosis or pulmonary embolism)
- Known inherited thrombophilic disorders (e.g. protein C, protein S, or antithrombin deficiency)
- Active or recent arterial thromboembolic disease (e.g. angina or myocardial infarction)
- Acute liver disease or unresolved liver dysfunction
- Porphyria
- Hypersensitivity to the active ingredients or any excipients
✔️ Pregnancy & Lactation
Femoston is not indicated during pregnancy. If pregnancy occurs during treatment, the medication should be discontinued immediately. Available epidemiological data on accidental exposure to oestrogen-progestogen combinations do not indicate an increased risk of congenital abnormalities or foetal toxicity. However, data specific to oestradiol/dydrogesterone use in pregnancy are limited.
Femoston is also not recommended during breastfeeding.
✔️ Precautions & Warnings
Before starting Femoston 2/10 Combipack Tablet, inform your doctor if you have any allergies to its components. The medicine should not be used during pregnancy or lactation and is not recommended for children.
Patients with premature menopause should discuss potential risks and benefits with their doctor before starting therapy. Avoid Femoston if you have a history of stroke, heart attack, angina, liver disease, or untreated thickening of the uterine lining.
If you have elevated blood triglyceride levels, please inform your doctor before treatment. Alcohol consumption should be avoided, as it may increase the risk of breast cancer.
Seek immediate medical attention if you experience symptoms such as sudden chest pain, breathing difficulty, or painful swelling and redness in the legs, as these may indicate blood clots.
Long-term use or high doses of Femoston may increase the risk of cardiovascular events, thromboembolism, breast cancer, or uterine cancer. These risks should be carefully discussed with your doctor before initiating treatment.
✔️ Storage:
Store in a cool and dry place away from sunlight.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.