✔ 100% Authentic Product
👁️ Currently Viewing 4147
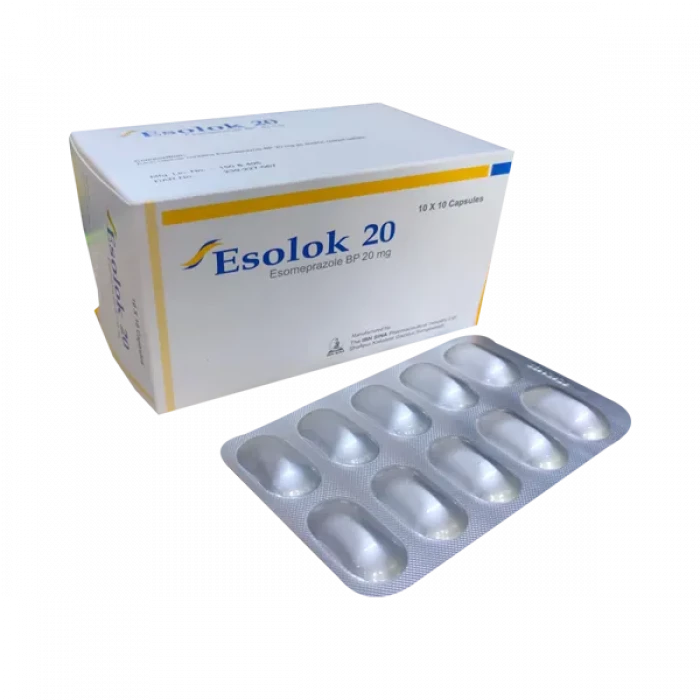
Esolok 20mg Tablet 10Pcs
Tablet Generic Name Esomeprazole Magnesium Trihydrate 20 mg Ibn Sina Pharmaceuticals Ltd.
Discount
Price: ৳ 48
MRP:
৳
50
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Indications of Esolok 20 Tablet
Esomeprazole is indicated for the Treatment of Gastroesophageal Reflux Disease (GERD) Healing of erosive esophagitis Maintenance of healing of erosive esophagitis Symptomatic Gastroesophageal Reflux Disease (GERD) Reduction of NSAID-associated gastric ulcer H. pylori eradication (Triple therapy) Zollinger-Ellison syndrome.
Pharmacology of Esolok 20 Tablet
Esomeprazole exerts its stomach acid-suppressing effects by preventing the final step in gastric acid production by covalently binding to sulfhydryl groups of cysteines found on the H+/K+-ATPase enzyme at the secretory surface of gastric parietal cells. This effect leads to the inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. As the binding of esomeprazole to the H+/K+-ATPase enzyme is irreversible and a new enzyme needs to be expressed in order to resume acid secretion, esomeprazole's duration of antisecretory effect that persists longer than 24 hours
Dosage of Esolok 20 Tablet
Tablet or Capsule: Healing of erosive esophagitis: 20 mg or 40 mg once daily for 4 to 8 weeks. The majority of patients are healed within 4 to 8 weeks. For patients who do not heal after 4-8 weeks, an additional 4-8 weeks of treatment may be considered. Maintenance of healing of erosive esophagitis: 20 mg once daily. Controlled studies did not extend beyond six months. Symptomatic GERD: 20 mg once daily for 4 weeks. If symptoms do not resolve completely after 4 weeks, an additional 4 weeks of treatment may be considered. H.pylori eradication (Triple therapy): Esomeprazole 20 mg twice daily for 10 days, Amoxicillin 1000 mg Twice daily for 10 days, and Clarithromycin 500 mg twice daily for 10 days Injection: The recommended adult dose in GERD with Erosive Esophagitis is either 20 or 40 mg Esomeprazole given once daily by intravenous injection (no less than 3 minutes) or intravenous infusion (10 to 30 minutes).
Administration of Esolok 20 Tablet
A solution for intravenous infusion is prepared by first reconstituting the contents of one vial with 5 ml of 0.9% Sodium Chloride and further diluting the resulting solution to a final volume of 50 ml. The resultant concentration after diluting to a final volume of 50 ml is 0.8 mg/ml. 20 mg dose: Withdraw 25 ml of the final solution and administer as an intravenous infusion over 10 minutes to 30 minutes. 10 mg dose: Withdraw 12.5 ml of the final solution and administer as an intravenous infusion over 10 minutes to 30 minutes.
Interaction of Esolok 20 Tablet
Esomeprazole appears to be a selective inhibitor of the cytochrome P450 mono-oxygenase system, there may be an effect on hepatic clearance, but there have been no reports to date of clinically relevant interactions. There is some uncertainty over the effect of Esomeprazole on the oral combined contraceptive pill. Physiological changes similar to those found with omeprazole are likely to take place because of the reduction in gastric acid which is likely to influence the bacterial colonization of the stomach and duodenum and also vitamin B12 absorption.
Contraindications
Esomeprazole is contraindicated in those patients who have known hypersensitivity to any other components of the formulation.
Side Effects of Esolok 20 Tablet
Side effects reported with Esomeprazole include headache, diarrhea, and abdominal pain. Pregnancy & Lactation US FDA pregnancy category of Esomeprazole is C. So, Esomeprazole should be avoided in pregnancy and lactation unless the potential benefits to the other outweigh the possible risks to the fetus.
Precautions & Warnings
Exclude the possibility of malignancy when gastric ulcer is suspected and before treatment for dyspepsia. When used in combination with antibiotics, refer to the prescribing information of the respective antibiotics. Reconstitution Directions for reconstitution of solution: The solution for injection is prepared by adding 5 ml of 0.9% Sodium Chloride for intravenous injection into the vial containing the dry powder. The reconstituted solution for injection is clear and colorless to very slightly yellow.
Storage Conditions
Keep in a dry place and away from light and heat. Keep out of the reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.