✔ 100% Authentic Product
👁️ Currently Viewing 2512
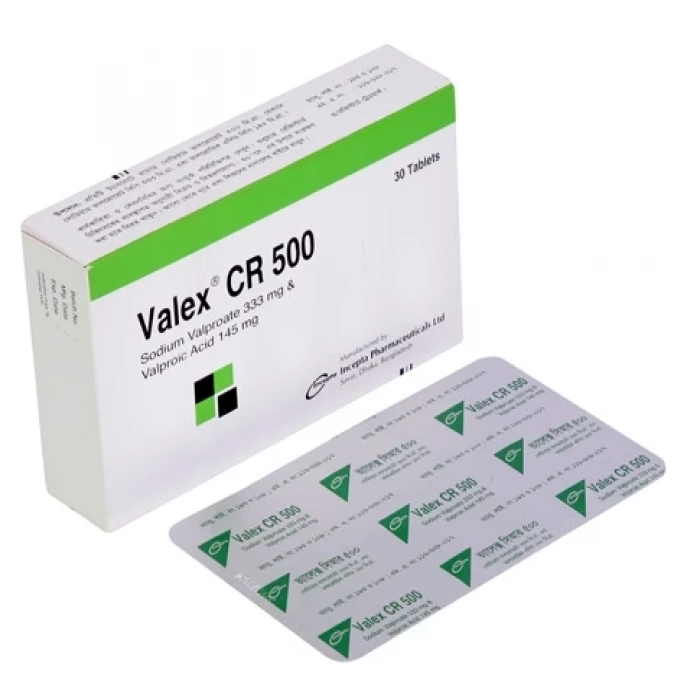
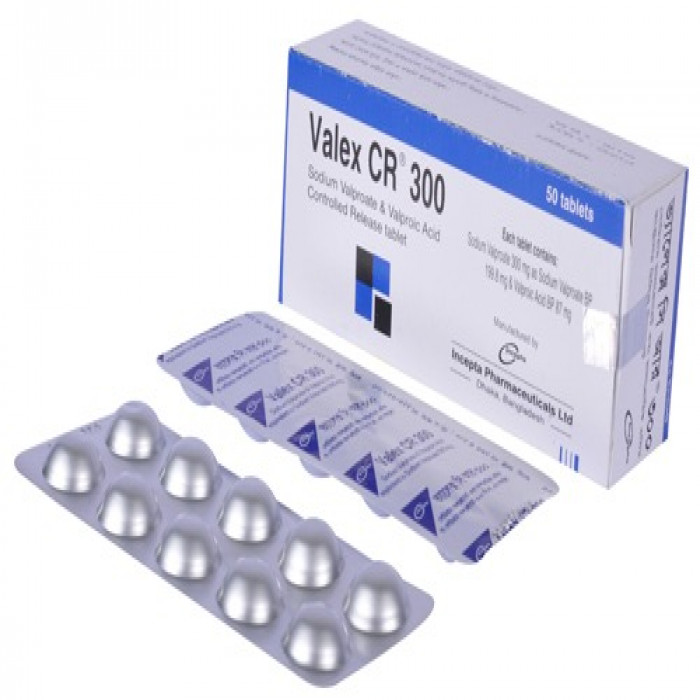
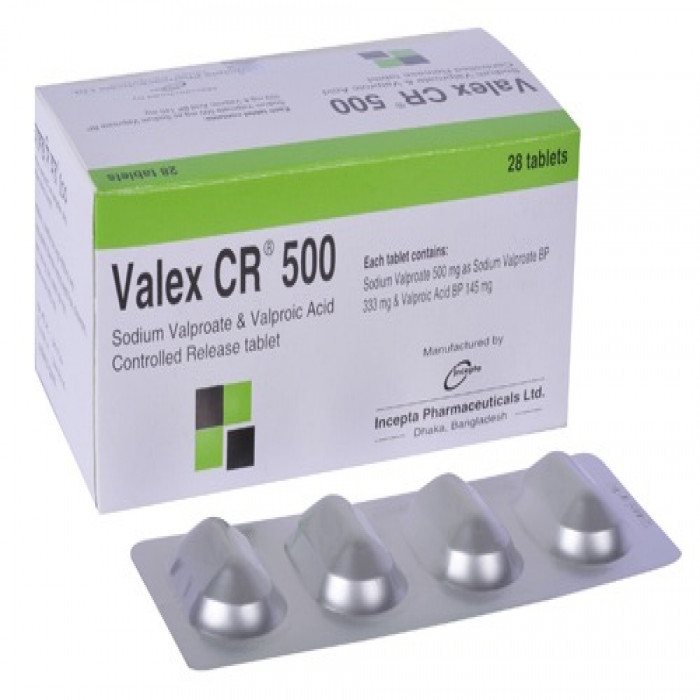
Valex CR 500mg 10pcs
Discount
Price: ৳ 124
MRP:
৳
130
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Indications
Oral sodium vaproate is approved for the treatment of all forms of epilepsy, including partial seizures.
Seizures in parts
Seizures caused by a lack of presence (petit mal)
Seizures with generalized tonic-clonic convulsions (grand mal)
Seizures that are myoclonic
Seizures that are atonic
Seizures that involve an absence attack are classified as mixed seizures.
Feverish convulsion prophylaxis
Post-traumatic epilepsy prophylaxis.
It is also used to treat bipolar illness and as a migraine preventative.
Pharmacology
The active component in this formulation, sodium valproate, has anti-epileptic efficacy against a variety of seizures. The method by which Sodium Valproate exerts its anti-epileptic properties is unknown. However, it has been proposed that its action is linked to increased gamma-aminobutyric acid levels in the brain (GABA).
Dosage & Administration
Sodium Valproate tablets may be given once or twice daily. Sodium Valproate syrup should be given in divided doses.
Epilepsy-
Adults: Initially 600 mg daily given in 2 divided doses, preferably after food, increasing by 200 mg/day at 3-day intervals to a maximum of 2.5 g daily in divided doses until control of seizure is achieved. Usual maintenance dose is 1-2 g daily (20-30 mg/kg daily)
Children (up to 20 kg): Initially 20 mg/kg daily in divided doses, may be increased provided plasma concentrations monitored (above 40 mg/kg daily also monitor clinical chemistry and hematological parameters).
Children (over 20 kg): Initially 400 mg daily in divided doses increased until control (usually in the range of 20-30 mg/kg daily) Maximum 35 mg/kg daily.
Febrile convulsion: 20-30 mg/kg/day in 3 divided doses.
Bipolar disorder: Initially 20-30 mg/kg/day in 2-3 divided doses; adjust dosage in 3-5 days. Maintenance dosage is 1000-2000 mg/day.
Prophylaxis of migraine: 300 mg twice daily, although some may require 1000 mg daily.
Interaction
Sodium vaproate appears to be a nonspecific inhibitor of drug metabolism. It has the most significant interactions with drugs such as phenobarbital, phenytoin, warfarin, aspirin, and others.
Contraindications
Sodium Valproate is not recommended for people who have a history of medication hypersensitivity or liver disease. Sodium Valproate is not recommended for use during pregnancy or in women of childbearing age.
Side Effects
Anorexia, nausea, and vomiting are the most prevalent adverse effects. These adverse effects, however, are reduced when enteric coated pills are used. Sedation, ataxia, and tremor are among the CNS effects. These symptoms occur infrequently and typically respond to a dosage reduction. Rash, alopecia, and appetite stimulation have been reported on occasion. Sodium vaproate has numerous impacts on hepatic function, including an increase in liver enzymes in plasma in up to 40% of patients, which frequently occurs asymptomatically during the first few months of medication. A potentially deadly fulminate hepatitis may occur in rare cases. Children under the age of two who have other medical problems or are being treated with numerous antiepileptic drugs are more vulnerable to hepatic damage. Acute pancreatitis and hyperammonemia have also been linked to the use of Sodium Valproate.
Pregnancy & Lactation
Sodium vaiproate passes the placenta, and in humans, first-trimester valproate exposure has been linked to neural tube abnormalities such as anencephaly and spina bifida in newborns. Pregnant women using Sodium Vaiproate should have their blood a-fetoprotein levels checked. Breast miik excretes sodium valproate. Breast-feeding by a mother taking Sodium Valproate, on the other hand, poses no harm to the kid.
Precautions & Warnings
Liver function should be evaluated before to medication and for the first 6 months, especially in patients who are at high risk. Before beginning and before major surgery, there must be no unnecessary risk of bleeding. Renal impairment, pregnancy, breast-feeding, and systemic lupus erythematosus should all be avoided. Because sodium valproate is partly removed in the urine as a ketone metabolite, the urine ketone test may be misinterpreted. It is best to prevent abrupt discontinuation of treatment. Sodium Valproate should not be taken during pregnancy or in women who are of childbearing age.
Storage Conditions
Store at temperatures no higher than 30°C. Keep away from light and out of children's reach.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.