✔ 100% Authentic Product
👁️ Currently Viewing 4192
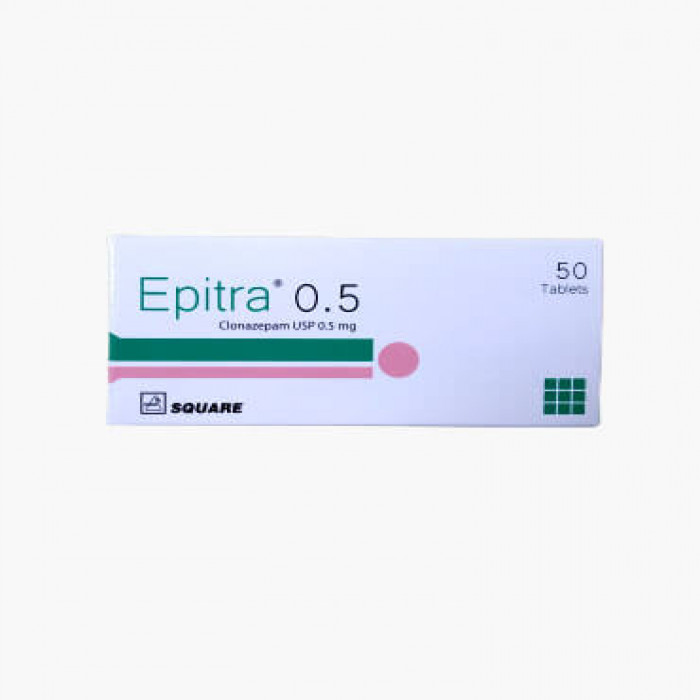
Epitra (Clonazepam) is indicated for the treatment of panic disorder, with or without agoraphobia. It is also used alone or as an adjunct in the treatment of Lennox-Gastaut Syndrome (petit mal variant), akinetic and myoclonic seizures. Additionally, it may be prescribed for patients with absence seizures (petit mal) who have not responded to succinimides.
📄Prescription Required
Discount
Price: ৳ 75
MRP:
৳
79
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Clonazepam
- Treatment of seizure disorder (epilepsy) in adults and children
- Management of panic disorder with or without agoraphobia in adults
- Including uncontrollable jerking or shaking, loss of consciousness, or collapsing.
- Characterized by sudden occurrence of panic attacks or fear.
During therapy, your doctor may conduct blood tests to assess liver function.
- Avoid alcohol consumption as it may exacerbate seizures and worsen side effects, leading to severe sedation.
- Not recommended for patients with myasthenia gravis, acute narrow-angle glaucoma, sleep apnea, lung diseases, breathing problems, or severe liver diseases.
- Use with caution in patients with mild to moderate liver disease, severe kidney disease, low blood pressure, depression, psychosis, schizophrenia, children, and elderly patients.
- Use in pregnant or breastfeeding women should be considered only if necessary, as it may pass through breast milk.
Always consult your doctor before taking Epitra 0.5mg Tablet, especially if you have any underlying medical conditions or concerns about potential side effects.
Safety Advices
Alcohol
UNSAFE
It's advised to abstain from alcohol consumption while on Epitra 0.5mg Tablet. Alcohol intake can trigger seizures, worsen side effects, and lead to severe sedation.
Pregnancy
UNSAFE
Epitra 0.5mg Tablet is generally not recommended during pregnancy unless deemed necessary by a doctor. Consult your healthcare provider before use.

Breastfeeding
UNSAFE
Avoid using Epitra 0.5mg Tablet while breastfeeding as it may pass into breast milk, potentially causing drowsiness and feeding difficulties. Consult your doctor before use.
Driving
CAUTION
Epitra 0.5mg Tablet may induce drowsiness or sleepiness, impairing your ability to drive or operate machinery. Avoid these activities while taking this medication.

Kidney
CAUTION
Use caution when taking Epitra 0.5mg Tablet in patients with severe kidney disease. Consult your doctor before use.
Liver
CAUTION
Epitra 0.5mg Tablet is not recommended for patients with severe liver disease. Use with caution in those with mild to moderate liver issues. Consult your doctor before use.
✔️ Uses of Epitra 0.5mg Tablet
Used to treat
- Seizures disorders
- Panic disorders
✔️ How does Uses of Epitra 0.5mg Tablet work?
Epitra 0.5mg Tablet belongs to benzodiazepines which work by enhancing the activity of GABA (gamma-aminobutyric acid) an inhibitory neurotransmitter (a chemical in the brain).
✔️ Side Effects of Uses of Epitra 0.5mg Tablet
Serious Side Effects:
- Contact your doctor immediately if experiencing suicidal thoughts, thoughts of self-harming, or unusual changes in behavior or mood.
Common Side Effects:
- Drowsiness, dizziness, coordination problems, depression, fatigue, memory problems.
- Contact your doctor if any symptoms worsen.
✔️ Quick Suggestions:
- Engage in regular exercise, as it can release endorphins and improve sleep quality and self-image, which helps lower anxiety levels.
- Find humor in your daily life by watching light-hearted shows or engaging in activities that make you laugh, as laughter can relieve stress.
- Practice mindfulness techniques such as yoga, meditation, mindfulness-based cognitive therapy, and mindfulness-based stress reduction to increase awareness and reduce anxiety.
- Stay hydrated by drinking enough water and limiting or avoiding alcohol and caffeine intake, as they can exacerbate anxiety symptoms.
- Follow a balanced diet rich in whole grains, vegetables, and fruits, and include anti-inflammatory herbs like turmeric, ginger, and chamomile to reduce inflammation associated with anxiety.
- Reduce intake of alcohol, caffeine, added sugar, high salt, and high-fat foods, especially trans fats, to help reduce inflammation.
- Incorporate antioxidants into your diet, such as ashwagandha, omega-3 fatty acids, green tea, and lemon, to promote overall well-being and reduce anxiety.
- Spend time with friends and family to build a strong social network, which can lower the risk of anxiety and provide support during challenging times.
- Take Epitra 0.5mg Tablet only in prescribed doses and as directed by your doctor to avoid dependence and abuse. Do not self-medicate.
- Be aware that Epitra 0.5mg Tablet may increase the risk of suicidal thoughts, so consult your doctor immediately if you notice any suicidal behavior.
✔️ Indication of Uses of Epitra 0.5mg Tablet
- Seizures: Seizures are sudden bursts of electrical activity in the brain, categorized into generalized seizures (affecting the entire brain) and partial seizures (affecting one part of the brain). They can cause uncontrollable muscle twitches and spasms, lasting from a few seconds to several minutes. Common triggers include lack of sleep, high fever, stress, bright lights, caffeine, certain medicines, alcohol, and irregular eating habits.
- Panic disorder: Panic disorder is an anxiety disorder characterized by frequent episodes of panic or dread, often without an apparent cause. While anxiety and panic are natural reactions to stressful situations, those with panic disorder experience these feelings regularly and intensely.
- Involuntary muscle spasms: Involuntary muscle spasms occur when nerve impulses controlling muscle movements are disrupted or damaged. Symptoms include muscle tightness, joint stiffness, abnormal posture, difficulty moving, and pain in affected muscles and joints. Triggers may include fatigue, stress, extreme temperatures, infections, and tight clothing.
- Restless leg syndrome (RLS): RLS is a nervous system disorder marked by an intense urge to move the legs, often accompanied by uncomfortable sensations like crawling or creeping. Symptoms worsen in the evening or at night, and may also affect the arms.
✔️ Pharmacology
Clonazepam, the active ingredient in Epitra, exhibits properties common to benzodiazepines, including anticonvulsive, sedative, muscle relaxing, and anxiolytic effects. It enhances GABAergic neurotransmission at inhibitory synapses, resulting in increased action of released GABA on postsynaptic transmembrane chloride ion flux. Clonazepam also affects serotonin and suppresses various types of paroxysmal activity, making it beneficial in generalized and focal epilepsies.
✔️ Dosage & Administration of Epitra 0.5mg Tablet
For adults with seizure disorders, the initial dose should not exceed 1.5 mg/day, divided into three doses. The dosage may be increased gradually until seizures are controlled, with a maximum daily dose of 20 mg. For panic disorder, the initial dose is 0.25 mg twice daily, with a target dose for most patients of 1 mg/day. Pediatric dosing should be individualized based on age and weight.
Overdose may cause drowsiness, ataxia, dysarthria, and respiratory depression. Supportive measures should be instituted as needed, and activated charcoal may be used to prevent further absorption. Flumazenil, a benzodiazepine antagonist, may be considered in severe cases.
✔️ Interaction
Epitra does not significantly alter the pharmacokinetics of certain drugs like phenytoin, carbamazepine, or phenobarbital. However, its effect on the metabolism of other drugs has not been fully investigated.
✔️ Contraindications
Epitra should not be used in patients with a history of hypersensitivity to benzodiazepines or with clinical or biochemical evidence of significant liver disease. It is contraindicated in acute narrow-angle glaucoma.
✔️ Pregnancy & Lactation
Clonazepam may cause congenital malformations and should be used during pregnancy only if the benefits outweigh the risks. It passes into breast milk in small amounts, so breastfeeding is not recommended during treatment.
✔️ Precaution & Warnings:
Epitra may increase the incidence of generalized tonic-clonic seizures in patients with multiple seizure disorders. Caution is advised when using it concurrently with valproic acid. In pediatric patients, increased salivation and bronchial secretions may occur. In geriatric patients, benzodiazepine effects may be greater due to age-related changes.
✔️ Storage Conditions:
Store Epitra in a dry place away from light and heat, and keep it out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.