✔ 100% Authentic Product
👁️ Currently Viewing 4894
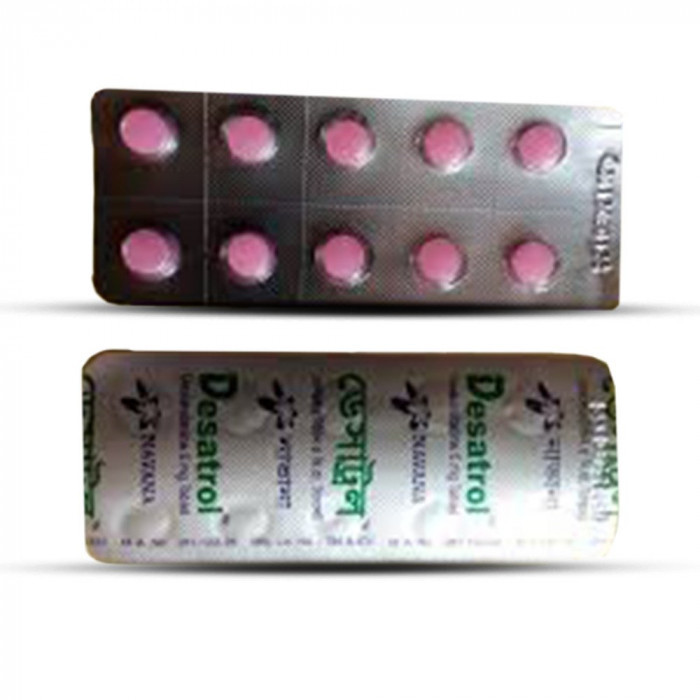
Desatrol 5mg 10pcs
Tablet, Manufacturer/Distributor: Navana Pharmaceuticals Ltd. Generic Name: Desloratadine 5 mg Tablet
Discount
Price: ৳ 24
MRP:
৳
25
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Desatrol Tablet: Each film coaled tablet contains Desloraladine BP 5 mg.
PHARMACOLOGY: Desatrol is the brand name of Desloratadine. Desloratadine is a long-acting tricyclic histamine antagonist with selective histamine1 (H1) receptor antagonist activity.
Receptor binding data indicates that at a concentration of 2-3 ng/ml (7nanomolar), Desloraladine shows significant interaction with the human hislamine1 (H1 )-receptor.
INDICATION: Allergic Rhinitis: Desatrol is indicated for the relief of the nasal and nonnasal symptoms of allergic rhinitis (seasonal and perennial).
Chronic idiopathic Urticaria: Desatrol is indicated for the symptomatic relief of pruritus, reduction in the number of hives and size of hives, in patients with chronic idiopathic urticaria.
DOSE AND ADMINISTRATION : Tablet : In adults and children 12 years of age and over, the recommended dose is 5 mg once daily.
In patients with liver or renal impairment, a starting dose of one 5 mg tablet every other day is recommended.
CONTRAINDICATION: Desatrol™ is contraindicated in patients who are hypersensitive to this medication or to any of its ingredients, or to loratadine.
WARNING AND PRECAUTION : Desloraladine should be used as directed. Patients should not increase dose or frequency as somnolence may occur. In general dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
SIDE EFFECTS : Desloraladine is well tolerated, non- sedative, long acting antihistamine and causes little or non-drowsiness. II has no muscarinic effect. Following adverse effects have been observed with Desloratadine : fatigue (3%), dry mouth (3%). dizziness (2%), nausea (2%), rarely tachycardia, hypersensitivity reactions. It does not cross the blood brain barrier.
USE IN PREGNANCY & LACTATION:
There are no adequate and well controlled studies in pregnant women, So, ii should be used during pregnancy only if clearly needed. Desloraladine appears in breast milk. Decision should be made whether to discontinue nursing or to discontinue Desloratadine, taking into account the importance of the drug to the mother.
USE IN CHILDREN & ADOLESCENTS :
The safety and effectiveness of Desloratadine in Children less than 6 months have not been established.
DRUG INTERACTION : Desloraladine 7.5 mg once daily was co-administered with erythromycin 500 mg every 8 hours or ketoconazole 200 mg every 12 hours for 10 days. Although increased plasma concentration of Desloratadine & 3- hydroxydesloratadine were observed, there were no clinically relevant changes in the safety profile of Desloratadine.
OVERDOSE: In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Desloratadine and 3-hyroxydesloratadine are not eliminated by hemodialysis.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.