✔ 100% Authentic Product
👁️ Currently Viewing 8846
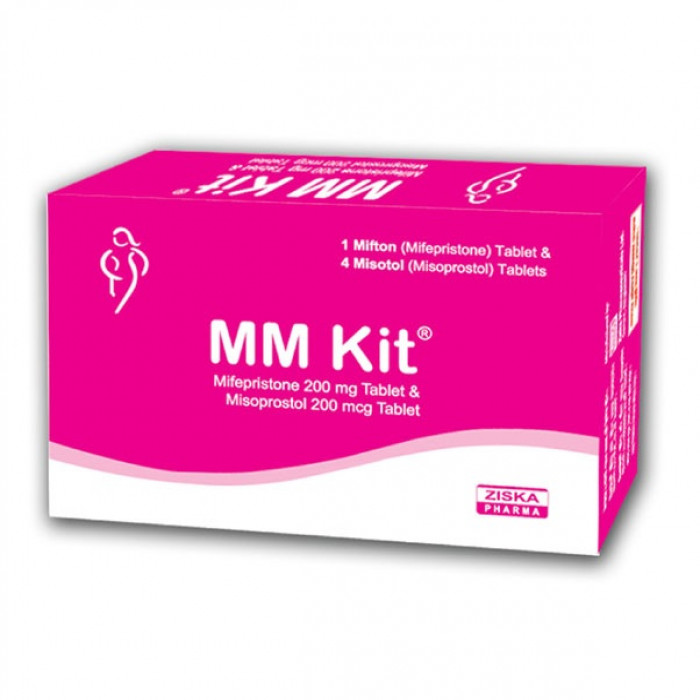
MM Kit is a safe and effective medical abortion kit indicated for early Menstrual Regulation (MR) and pregnancy termination up to 9 weeks (63 days) of gestation. It contains Mifepristone and Misoprostol, the globally trusted combination for early pregnancy abortion. This MM Kit is designed for non-surgical termination and is widely used for safe and private pregnancy management.
Discount
Price: ৳ 288
MRP:
৳
300
4%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
The MM Kit is a medical abortion kit used for early pregnancy termination up to 10 weeks (70 days) from the first day of your last menstrual period. It contains two essential medications: Mifepristone and Misoprostol, which work together to safely and effectively terminate a pregnancy.
How Does the MM Kit Work?
Mifepristone blocks the hormone progesterone, which is necessary for maintaining pregnancy. Without progesterone, the pregnancy cannot continue.
Misoprostol is taken 24-48 hours after Mifepristone and causes contractions to expel the pregnancy tissue.
Key Features of the MM Kit:
Safe and Effective: Clinically proven for early pregnancy termination up to 9 weeks (63 days).
Non-Surgical: No need for invasive procedures; the process is managed privately at home.
Globally Trusted: Used by healthcare providers worldwide for medical abortion.
Easy to Use: Each box contains 2 blister strips:
Mifton Strip: 1 tablet of Mifepristone 200 mg.
Misotol Strip: 4 tablets of Misoprostol 200 mcg each.
Important Safety Information:
Do not use the MM Kit if you have an ectopic pregnancy (a pregnancy outside the womb). It will not work in such cases and may cause severe complications.
Always consult a healthcare provider before using the MM Kit to ensure it is safe for you.
Why Choose the MM Kit?
High Success Rate: Over 95% effective when used correctly.
Privacy and Convenience: Manage your pregnancy termination in the comfort of your home.
Trusted Composition: Contains Mifepristone and Misoprostol, the gold standard for medical abortion.
Safety Advices

Alcohol
UNSAFE
It is unsafe to consume alcohol with MM-Kit.

Pregnancy
UNSAFE
MM-Kit is highly unsafe to use during pregnancy. Seek your doctor's advice as studies on pregnant women and animals have shown significant harmful effects on the developing baby.

Breastfeeding
UNSAFE
MM-Kit is unsafe to use during breastfeeding. Data suggests that the drug may cause toxicity to the baby.

Driving
UNSAFE
MM-Kit may decrease alertness, affect your vision or make you feel sleepy and dizzy. Do not drive if these symptoms occur.

Liver
CONSULT YOUR DOCTOR
There is limited information available on the use of MM-Kit in patients with kidney disease. Please consult your doctor.

Kidney
SAFE IF PRESCRIBED
MM-Kit is probably safe to use in patients with liver disease. Limited data available suggests that dose adjustment of MM-Kit may not be needed in these patients. Please consult your doctor.
✔️ Pharmacology
Mifton (Mifepristone): Mifepristone is a synthetic steroid with an anti-progestational activity that results from competitive interaction with progesterone at progesterone-receptor sites. Based on studies with various oral doses in several animals, species the compound inhibits the activity of endogenous or exogenous progesterone and the Menstrual Regulation (MR) results. During pregnancy, the compound sensitizes the myometrium to the contraction-inducing activity of prostaglandins.
Misotol (Misoprostol): Misoprostol is a synthetic analog of prostaglandin E1. It causes myometrial contraction by interacting with specific receptors on myometrial cells. This interaction results in a change in calcium concentration, thereby initiating muscle contraction. By interacting with prostaglandin receptors, Misoprostol causes the cervix to soften and the uterus to contract, resulting in the expulsion of the uterine contents.
✔️ Dosage & Administrations of MM Kit:
Only qualified medical professionals who can assess an embryo's gestational age and diagnose ectopic pregnancies can prescribe the MM Kit. In addition, qualified medical professionals must be able to provide surgical intervention/MVA (Manual Vacuum Aspiration) in cases of incomplete abortion or severe bleeding, or have plans in place to provide such care through others, and be able to ensure patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.
Day 1 (First visit): Mifepristone administration
- One tablet of Mifepristone (200 mg) is taken in a single oral dose under the supervision of a qualified medical professional in a clinic, medical office, or hospital.
Day 2 (Second visit): Misoprostol administration
- 24-48 hours after ingesting the Mifepristone tablet, the patient takes four 200-microgram tablets(800 micrograms) of Misoprostol buccally or sublingually. Misoprostol tablets can be administered by the patient herself (place two tablets on each side of the cheek & gum or under the tongue). She should wait for 30 minutes.
- During the period immediately following the administration of Misoprostol, the patient may need medication for cramps or gastrointestinal symptoms. The patient should be given instructions on what to do if significant discomfort, excessive bleeding, or other adverse reactions occur and should be given a phone number to call if she has questions following the administration of Misoprostol.
Day 10 to 14 (Third visit): post-treatment examination
- Patients must return to the clinic, medical office, or hospital within 10 to 14 days after the administration of mifepristone. This visit is very important to confirm by clinical examination or ultrasonography scan that a complete termination of pregnancy has occurred.
✔️ Uses of MM-Kit
- Medical Abortion
✔️ Quick Suggestions:
- The MM-Kit aids in the termination of a pregnancy.
- It can cause dizziness and sleepiness when driving or doing anything that requires concentration.
- It does not affect fertility. To prevent pregnancy, use contraception.
- Termination of pregnancy
✔️ MM KIT Side Effects
Mifton (Mifepristone): The treatment procedure is designed to induce vaginal bleeding and uterine cramping necessary for Menstrual Regulation (MR).
- Common side effects: Nausea/Vomiting and Diarrhea.
- Rare side effects: Pelvic pain, Fainting, Headache, Dizziness, and Asthenia
Misotol (Misoprostol): Gastro-intestinal side-effects like
- Diarrhea
- Abdominal pain
- Flatulence
- Dyspepsia
- Headache
- Vomiting and constipation
- Shivering
- Hyperthermia
- Dizziness
Pain due to uterine contractions, severe vaginal bleeding, shock, pelvic pain, and uterine rupture (requiring surgical repair, hysterectomy, and/or salpingo-oophorectomy).
✔️ How to use MM-Kit
Take this medicine in the dose and duration as advised by your doctor. Swallow it as a whole. Do not chew, crush or break it. MM-Kit is to be taken with food.
✔️ How does MM-Kit work?
MM-Kit is a combination of two medicines: Mifepristone and Misoprostol, which causes abortion. Mifepristone blocks the effects of progesterone, a natural female hormone that is needed for the pregnancy to sustain. Without this hormone, the lining of the uterus (womb) breaks down as it does during a menstrual period and stops the growth of the pregnancy. Mifepristone increases contractions of the uterus to cause abortion.
✔️ Use in Impairmnet patients:
Use in Patients
- With Hepatic Impairment: Misotol® (Misoprostol): Patients with hepatic disease should receive the decreased dose.
Use in Patients
- With Renal Impairment: Misotol® (Misoprostol): No routine dosage adjustment is recommended in older patients or patients with renal impairment but the dosage may need to be reduced if the usual dose is not tolerated.
✔️ Overdosage of MM KIT:
Mifton (Mifepristone): No serious adverse reactions were reported in tolerance studies in healthy nonpregnant female and healthy male subjects where Mifepristone was administered in single doses greater than threefold of 600mg for Menstrual Regulation (MR). If a patient ingests a massive overdose, she should be observed closely for signs of adrenal failure.
Misotol (Misoprostol): Clinical signs that may indicate an overdose are a sedation, tremor, convulsions, dyspnea, abdominal pain, diarrhea, fever, palpitations, hypotension, or bradycardia. Symptoms should be treated with supportive therapy. However, because Misoprostol is metabolized like a fatty acid, it is unlikely that dialysis would be an appropriate treatment for overdosage.
✔️ Use of MM Kit in Pregnancy and Lactation:
Pregnancy:
Mifton (Mifepristone):
- It is indicated for Menstrual Regulation (MR) (through 63 days of pregnancy) and has no other approved indication for use during pregnancy. Patients who have an ongoing pregnancy at the last visit have a risk of fetal malformation resulting from the treatment. Surgical termination is recommended to manage Menstrual Regulation (MR) treatment failures.
Lactation:
Mifton (Mifepristone):
- It is not known whether Mifepristone is excreted through human milk. Many hormones with a similar chemical structure, however, are excreted in breast milk. Since the effects of Mifepristone on infants are unknown, breastfeeding women should consult with their doctor to decide if they should discard their breastmilk for a few days following the administration of the medications.
Misotol (Misoprostol):
- Although it is not known whether Misoprostol or Misoprostol is excreted through human milk, Misoprostol should not be administered to nursing mothers because the potential excretion of misoprostol acid could cause diarrhea in nursing infants.
✔️ MM KIT Drug Interaction
Mifton (Mifepristone): Although specific drug or food interactions with Mifepristone have not been studied, on the basis of this drug's metabolism by CYP 3A4, it is possible that Ketoconazole, Itraconazole, Erythromycin, and grapefruit juice may inhibit its metabolism (increasing serum levels of mifepristone).
Misotol® (Misoprostol): Misoprostol has not been shown to interfere with the beneficial effects of aspirin on signs and symptoms of rheumatoid arthritis. Misoprostol does not exert clinically significant effects on the absorption, blood levels, and antiplatelet effects of therapeutic doses of aspirin.
✔️ Contraindication
Administration of Mifepristone is contraindicated in patients with any one of the following conditions: History of allergy or known hypersensitivity to Mifepristone, Misoprostol or another prostaglandin, confirmed or suspected ectopic pregnancy or undiagnosed adnexal mass (the treatment procedure will not be effective to terminate an ectopic pregnancy), IUD in place, chronic adrenal failure, hemorrhagic disorders or concurrent anticoagulant therapy, inherited porphyria, If a patient does not have adequate access to medical facilities equipped to provide emergency treatment of incomplete process, blood transfusions, and emergency resuscitation during the period from the first visit until discharged by the administering physician.
✔️ Precaution and Warnings:
- The patient should not give a combination of Mifepristone & Misoprostol to anyone else.
- The combination of Mifepristone & Misoprostol has been prescribed for the patient's specific condition, it may not be the correct treatment for another person, and may be dangerous to the other person if she is or were to become pregnant. Any Intra Uterine Device [IUD] should be removed before treatment with Mifepristone begins.
- Menstrual Regulation (MR) by surgery is recommended in cases when a combination of Mifepristone & Misoprostol fails to cause Menstrual Regulation (MR).
- Patients who have an ongoing pregnancy at the last visit have a risk of fetal malformation resulting from the treatment.
- Surgical termination/MVA is recommended to manage Menstrual Regulation (MR)/ termination of pregnancy failures.
✔️ MM KIT Storage
- Store the MM KIT at room temperature in a clean and dry place, protected from moisture, sunlight, and heat.
- Keep the medicine away from children and pets.
✔️ Disposal of MM KIT
Discard any unused medicine properly. Do not flush it in the toilet or throw it into the drain.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.